| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:40:48 UTC |
|---|
| Update Date | 2016-11-09 01:19:01 UTC |
|---|
| Accession Number | CHEM030137 |
|---|
| Identification |
|---|
| Common Name | Anabsin |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Artemisia absinthium (wormwood). Anabsin is found in alcoholic beverages and herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
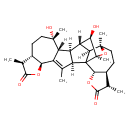
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C30H40O7 |
|---|
| Average Molecular Mass | 512.634 g/mol |
|---|
| Monoisotopic Mass | 512.277 g/mol |
|---|
| CAS Registry Number | 72542-39-3 |
|---|
| IUPAC Name | (2R,5S,8S,9S,12S,13R,14R,15S,16R,17S,19R,22S,23S,26S,27R)-12,16-dihydroxy-3,8,12,17,19,23-hexamethyl-6,18,25-trioxaoctacyclo[13.11.1.0¹,¹⁷.0²,¹⁴.0⁴,¹³.0⁵,⁹.0¹⁹,²⁷.0²²,²⁶]heptacos-3-ene-7,24-dione |
|---|
| Traditional Name | (2R,5S,8S,9S,12S,13R,14R,15S,16R,17S,19R,22S,23S,26S,27R)-12,16-dihydroxy-3,8,12,17,19,23-hexamethyl-6,18,25-trioxaoctacyclo[13.11.1.0¹,¹⁷.0²,¹⁴.0⁴,¹³.0⁵,⁹.0¹⁹,²⁷.0²²,²⁶]heptacos-3-ene-7,24-dione |
|---|
| SMILES | [H][C@]12C[C@@](C)(O)C[C@H]3[C@H](C)C(=O)O[C@@H]3C1=C(C)[C@@]1([H])[C@]2([H])[C@@]2([H])[C@H](O)[C@@]3(C)O[C@@]4(C)CC[C@H]5[C@H](C)C(=O)O[C@@H]5C13[C@@]24[H] |
|---|
| InChI Identifier | InChI=1S/C30H40O7/c1-11-14-7-8-28(5)22-19-18-16-10-27(4,34)9-15-12(2)25(32)35-21(15)17(16)13(3)20(18)30(22,24(14)36-26(11)33)29(6,37-28)23(19)31/h11-12,14-16,18-24,31,34H,7-10H2,1-6H3/t11-,12-,14-,15-,16-,18-,19+,20-,21-,22-,23-,24-,27-,28-,29+,30?/m0/s1 |
|---|
| InChI Key | ISHUGAFOZRITCZ-VYRXGUTMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesterterpenoids. These are terpenes composed of five consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesterterpenoids |
|---|
| Direct Parent | Sesterterpenoids |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-0013900000-7ffa92660086a215a341 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01oy-1001079000-172ba852ee025ebc8d4a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Anabsin,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0000930000-4581653e04cd8ab94213 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00tk-0000900000-758c6b49482a2731fe3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-2032900000-8a09e5eb96eab9ceb3f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0000890000-d75e001acd1d21e09026 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02tc-0000920000-812e843c652a6cfbf480 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0h91-3001900000-fb1dc022cff29494b3e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-c70f3135f614c244e1c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000090000-0f464e2af1d28348dfd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fu-2000930000-ccdf9ff1c73be2a84264 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000190000-cd6f0688015ce67ab60e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-0000950000-51c2eac3a17325f2243d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-1111950000-f7f62c21c42aadbd3cae | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036414 |
|---|
| FooDB ID | FDB015296 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020968 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|