| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:39:50 UTC |
|---|
| Update Date | 2016-11-09 01:19:01 UTC |
|---|
| Accession Number | CHEM030116 |
|---|
| Identification |
|---|
| Common Name | N-cis-Feruloyltyramine |
|---|
| Class | Small Molecule |
|---|
| Description | N-cis-Feruloyltyramine is found in cherimoya. N-cis-Feruloyltyramine is isolated from bell pepper. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
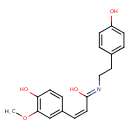
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| cis-N-Feruloyltyramine | HMDB | | N-cis-Feruloyl tyramine | HMDB | | (2Z)-3-(4-Hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enimidate | HMDB | | N-cis-Feruloyltyramine | HMDB | | (2,3) cis-N-(P-Hydroxyphenethyl)ferulamide | HMDB | | (2Z)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]-2-propenamide | HMDB | | (2Z)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enamide | HMDB | | Feruloyltyramine | HMDB | | Feruloyltyramine, (Z)-isomer | HMDB | | NCT | HMDB | | N-[(Z)-Feruloyl]tyramine | HMDB | | N-Feruloyltyramine | HMDB | | 3-(4-hydroxy-3-methoxyphenyl)-N-(2-(4-hydroxyphenyl)ethyl)-2-Propenamide | HMDB |
|
|---|
| Chemical Formula | C18H19NO4 |
|---|
| Average Molecular Mass | 313.348 g/mol |
|---|
| Monoisotopic Mass | 313.131 g/mol |
|---|
| CAS Registry Number | 80510-09-4 |
|---|
| IUPAC Name | (Z,2Z)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]propa-2-enimidic acid |
|---|
| Traditional Name | (Z,2Z)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]propa-2-enimidic acid |
|---|
| SMILES | COC1=C(O)C=CC(\C=C/C(/O)=N/CCC2=CC=C(O)C=C2)=C1 |
|---|
| InChI Identifier | InChI=1S/C18H19NO4/c1-23-17-12-14(4-8-16(17)21)5-9-18(22)19-11-10-13-2-6-15(20)7-3-13/h2-9,12,20-21H,10-11H2,1H3,(H,19,22)/b9-5- |
|---|
| InChI Key | NPNNKDMSXVRADT-UITAMQMPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxycinnamic acids and derivatives. Hydroxycinnamic acids and derivatives are compounds containing an cinnamic acid (or a derivative thereof) where the benzene ring is hydroxylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Hydroxycinnamic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid amide
- Hydroxycinnamic acid or derivatives
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Styrene
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056s-1930000000-a57a34515fcd6d29f561 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-044i-2303390000-593dc157920ba7f3729f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p9-0902000000-35cbd3fbcb8058d64329 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0900000000-714a65fcbd5aa8249bea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0avs-3900000000-cdd8344e1c4c188399fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0419000000-87271f4c26fc442256b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08ic-0922000000-64a362d838af2e86e0dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-3900000000-b725e376ed0d4ee8b405 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0029000000-f45d1232bf98a8734c0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-0934000000-58c76ec993e92bd840d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1974000000-f0358dabe0c9f7fc6cdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0129000000-31dd37ff9949311c7f49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0239-0942000000-e2db54ed7422a2be9f9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2900000000-85b2762db28b2dda88d3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036381 |
|---|
| FooDB ID | FDB015259 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00025324 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4944914 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6440659 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|