| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:38:21 UTC |
|---|
| Update Date | 2016-11-09 01:19:00 UTC |
|---|
| Accession Number | CHEM030077 |
|---|
| Identification |
|---|
| Common Name | 15-Oleoylsolamin |
|---|
| Class | Small Molecule |
|---|
| Description | 15-Oleoylsolamin is found in fruits. 15-Oleoylsolamin is a constituent of Annona muricata (soursop) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
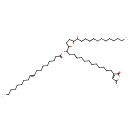
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 15-(9-Octadecenoyl)solamin | HMDB | | Amanil fast yellow BX | HMDB | | Atlantic fast yellow FFX | HMDB | | Benzanil supra yellow GX | HMDB | | C.I. direct yellow 29 | HMDB | | C.I. direct yellow 29, disodium salt (8ci) | HMDB | | Chloramine yellow g | HMDB | | Chloramine yellow R | HMDB | | Chrome leather yellow BRL | HMDB | | Chrome leather yellow or | HMDB | | Cuprodiazol light yellow JR | HMDB | | Diamine supra yellow RT | HMDB | | Diaphtamine fast yellow b | HMDB | | Diazol fast yellow b | HMDB | | Diazol light yellow jma | HMDB | | Direct fast light yellow b | HMDB | | Direct fast yellow b | HMDB | | Direct yellow 29 | HMDB | | Durazol paper yellow GR | HMDB | | Durazol yellow | HMDB | | Durazol yellow GR | HMDB | | Durazol yellow GRP | HMDB | | Eliamina yellow RT | HMDB | | Enianil fast yellow b | HMDB | | Enianil light yellow RT | HMDB | | Fastusol yellow LRTP-CF | HMDB | | Fenaluz yellow 2R | HMDB | | Helion yellow BRL | HMDB | | Hispaluz yellow b | HMDB | | Kca light fast yellow RT | HMDB | | Oxyphenine R | HMDB | | Pontamine fast yellow BBL | HMDB | | Saturn yellow LRT | HMDB | | Sirius supra yellow RT | HMDB | | Solar yellow b | HMDB | | Solius light yellow RT | HMDB | | Solophenyl yellow BRL | HMDB | | Solophenyl yellow FL | HMDB | | Tertrodirect fast yellow RT | HMDB | | Tertrodirect yellow CX | HMDB | | Tetramine fast yellow WB extra | HMDB | | Triantine fast yellow RT | HMDB | | Triantine light yellow RT | HMDB | | 1-[5-(1-Hydroxytridecyl)oxolan-2-yl]-13-(5-methyl-2-oxo-2,5-dihydrofuran-3-yl)tridecyl (9E)-octadec-9-enoic acid | Generator |
|
|---|
| Chemical Formula | C53H96O6 |
|---|
| Average Molecular Mass | 829.326 g/mol |
|---|
| Monoisotopic Mass | 828.721 g/mol |
|---|
| CAS Registry Number | 213313-62-3 |
|---|
| IUPAC Name | 1-[5-(1-hydroxytridecyl)oxolan-2-yl]-13-(5-methyl-2-oxo-2,5-dihydrofuran-3-yl)tridecyl (9E)-octadec-9-enoate |
|---|
| Traditional Name | 1-[5-(1-hydroxytridecyl)oxolan-2-yl]-13-(5-methyl-2-oxo-5H-furan-3-yl)tridecyl (9E)-octadec-9-enoate |
|---|
| SMILES | CCCCCCCCCCCCC(O)C1CCC(O1)C(CCCCCCCCCCCCC1=CC(C)OC1=O)OC(=O)CCCCCCC\C=C\CCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C53H96O6/c1-4-6-8-10-12-14-16-17-18-19-20-26-30-34-38-42-52(55)59-50(41-37-33-29-25-22-21-23-27-31-35-39-47-45-46(3)57-53(47)56)51-44-43-49(58-51)48(54)40-36-32-28-24-15-13-11-9-7-5-2/h17-18,45-46,48-51,54H,4-16,19-44H2,1-3H3/b18-17+ |
|---|
| InChI Key | CYNQUXCKSVIOBA-ISLYRVAYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as annonaceous acetogenins. These are waxy derivatives of fatty acids (usually C32 or C34), containing a terminal carboxylic acid combined with a 2-propanol unit at the C-2 position to form a methyl- substituted alpha,beta-unsaturated-gamma-lactone. One of their interesting structural features is a single, adjacent, or nonadjacent tetrahydrofuran (THF) or tetrahydropyran (THP) system with one or two flanking hydroxyl group(s) at the center of a long hydrocarbon chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Annonaceous acetogenins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Annonaceae acetogenin skeleton
- Long chain fatty alcohol
- Fatty alcohol ester
- Fatty acid ester
- 2-furanone
- Dicarboxylic acid or derivatives
- Dihydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Tetrahydrofuran
- Enoate ester
- Lactone
- Carboxylic acid ester
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00os-0160061390-e880f7bd05a1761cb1c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0390020710-60aeb3804e75821f6ade | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-0241020900-fda4d59d902cb50772bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-0050040390-8f687a6bf7476dcff989 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03yi-1291082140-fe39002c05ffc03ef309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-029x-2091070000-75501a1ca88053e87476 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-1120021190-613aef645af8821c2c85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-1000000490-1c3a876807391455869f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9500010100-bc9eb322c6581755dcef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0020001090-cc4fe46a061700bd537b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-5093013280-4890d11267e6730a29bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0900-9251000010-d901d28d54bf70fd7ead | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036342 |
|---|
| FooDB ID | FDB015213 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057247 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014119 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751967 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|