| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:36:18 UTC |
|---|
| Update Date | 2016-11-09 01:18:59 UTC |
|---|
| Accession Number | CHEM030020 |
|---|
| Identification |
|---|
| Common Name | Campesteryl ferulate |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of various cereal grains
26Mg has found application in isotopic geology, similar to that of aluminium. 26Mg is a radiogenic daughter product of 26Al, which has a half-life of 717,000 years. Large enrichments of stable 26Mg have been observed in the Ca-Al-rich inclusions of some carbonaceous chondrite meteorites. The anomalous abundance of 26Mg is attributed to the decay of its parent 26Al in the inclusions. Therefore, the meteorite must have formed in the solar nebula before the 26Al had decayed. Hence, these fragments are among the oldest objects in the solar system and have preserved information about its early history.; Elemental magnesium is a fairly strong, silvery-white, light-weight metal (two thirds the density of aluminium). It tarnishes slightly when exposed to air, although unlike the alkaline metals, storage in an oxygen-free environment is unnecessary because magnesium is protected by a thin layer of oxide which is fairly impermeable and hard to remove. Like its lower periodic table group neighbor calcium, magnesium reacts with water at room temperature, though it reacts much more slowly than calcium. When it is submerged in water, hydrogen bubbles will almost unnoticeably begin to form on the surface of the metal, though if powdered it will react much more rapidly. The reaction will occur faster with higher temperatures (see precautions). Magnesium also reacts exothermically with most acids, such as hydrochloric acid (HCl). As with aluminium, zinc and many other metals, the reaction with hydrochloric acid produces the chloride of the metal and releases hydrogen gas.; Historically, magnesium was one of the main aerospace construction metals and was used for German military aircraft as early as World War I and extensively for German aircraft in World War II. The Germans coined the name 'Elektron' for magnesium alloy which is still used today. Due to perceived hazards with magnesium parts in the event of fire, the application of magnesium in the commercial aerospace industry was generally restricted to engine related components. Currently the use of magnesium alloys in aerospace is increasing, mostly driven by the increasing importance of fuel economy and the need to reduce weight. The development and testing of new magnesium alloys continues, notably Elektron 21 which has successfully undergone extensive aerospace testing for suitability in engine, internal and airframe components. The European Community runs three R&D magnesium projects in the Aerospace priority of Six Framework Program.; Magnesium (pronounced /mæ??ni?zi?m/) is a chemical element with the symbol Mg, atomic number 12, atomic weight 24.3050 and common oxidation number +2.; Magnesium compounds are typically white crystals. Most are soluble in water, providing the sour-tasting magnesium ion Mg2+. Small amounts of dissolved magnesium ion contributes to the tartness and taste of natural waters. Magnesium ion in large amounts is an ionic laxative, and magnesium sulfate (Epsom salts) is sometimes used for this purpose. So-called "milk of magnesia" is a water suspension of one of the few insoluble magnesium compounds, magnesium hydroxide. The undissolved particles give rise to its appearance and name. Milk of magnesia is a mild base commonly used as an antacid.; Magnesium is a highly flammable metal, but while it is easy to ignite when powdered or shaved into thin strips, it is difficult to ignite in mass or bulk. Once ignited, it is difficult to extinguish, being able to burn in both nitrogen (forming magnesium nitride), and carbon dioxide (forming magnesium oxide and carbon). On burning in air, magnesium produces a brilliant white light. Thus magnesium powder (flash powder) was used as a source of illumination in the early days of photography. Later, magnesium ribbon was used in electrically ignited flash bulbs. Magnesium powder is used in the manufacture of fireworks and marine flares where a brilliant white light is required. Flame temperatures of magnesium and magnesium alloys can reach 1,371 °C (2,500 °F), although flame height above the burning metal is usually less than 300 mm (12 in).; Magnesium is a vital component of a healthy human diet. Human magnesium deficiency (including conditions which show few overt symptoms) is relatively common, with only 32% of the United States meeting the RDA-DRI, and has been implicated in the development of a number of human illnesses such as asthma, osteoporosis, and ADHD.; Magnesium salts are essential in nutrition, being required for the activity of many enzymes, especially those concerned with oxidative phosphorylation. Physiologically, it exists as an ion in the body. It is a component of both intra- and extracellular fluids and is excreted in the urine and feces. Deficiency causes irritability of the nervous system with tetany, vasodilatation, convulsions, tremors, depression, and psychotic behavior. Magnesium ion in large amounts is an ionic laxative, and magnesium sulfate (Epsom salts) is sometimes used for this purpose. So-called "milk of magnesia" is a water suspension of one of the few insoluble magnesium compounds, magnesium hydroxide; There are a number of magnesium dietary supplements available. Magnesium oxide, one of the most common because it has a high magnesium content per weight, has been reported to be the least bioavailable. Magnesium citrate has been reported as more bioavailable than oxide or amino-acid chelate (glycinate) forms.; the undissolved particles give rise to its appearance and name. Milk of magnesia is a mild base, and is commonly used as an antacid. Campesteryl ferulate is found in cereals and cereal products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
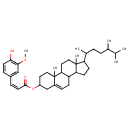
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Campesteryl ferulic acid | Generator | | 14-(5,6-Dimethylheptan-2-yl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl (2Z)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | Generator |
|
|---|
| Chemical Formula | C38H56O4 |
|---|
| Average Molecular Mass | 576.849 g/mol |
|---|
| Monoisotopic Mass | 576.418 g/mol |
|---|
| CAS Registry Number | 20972-07-0 |
|---|
| IUPAC Name | 14-(5,6-dimethylheptan-2-yl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl (2Z)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| Traditional Name | 14-(5,6-dimethylheptan-2-yl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl (2Z)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| SMILES | COC1=C(O)C=CC(\C=C/C(=O)OC2CCC3(C)C4CCC5(C)C(CCC5C4CC=C3C2)C(C)CCC(C)C(C)C)=C1 |
|---|
| InChI Identifier | InChI=1S/C38H56O4/c1-24(2)25(3)8-9-26(4)31-14-15-32-30-13-12-28-23-29(18-20-37(28,5)33(30)19-21-38(31,32)6)42-36(40)17-11-27-10-16-34(39)35(22-27)41-7/h10-12,16-17,22,24-26,29-33,39H,8-9,13-15,18-21,23H2,1-7H3/b17-11- |
|---|
| InChI Key | SWIWTAJTJOYCTB-BOPFTXTBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Delta-5-steroid
- Hydroxycinnamic acid or derivatives
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Cinnamic acid ester
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Carboxylic acid derivative
- Ether
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08i4-2309170000-f0c04e704762f2eba062 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-053u-3019017000-6699aac253ab20639127 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Campesteryl ferulate,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1503390000-c72c3581076d2b4bcd19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-4917320000-43fbf760d88dc4b64fb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-5149010000-bdcefdc34d8109caac12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0204090000-978a7dadfbfcd16657c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0709140000-878d44c43536f944f7c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001j-1409000000-67e7486000086b06ea9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0001090000-a4d6aad6b6f094546363 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05ia-0401290000-b3fa08b0aa6800778fe1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fl0-0300190000-f59bb213b81380f3aad8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000190000-fdd4094930e15e1ef7cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9322120000-e05107b2755327ef3332 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05al-9631000000-44423afe0fb291427703 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036285 |
|---|
| FooDB ID | FDB015151 |
|---|
| Phenol Explorer ID | 560 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Γ-Oryzanol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751942 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|