| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:33:11 UTC |
|---|
| Update Date | 2016-11-09 01:18:59 UTC |
|---|
| Accession Number | CHEM029966 |
|---|
| Identification |
|---|
| Common Name | Furoeremophilone 1 |
|---|
| Class | Small Molecule |
|---|
| Description | Furoeremophilone 1 is found in green vegetables. Furoeremophilone 1 is a constituent of Smyrnium olusatrum (alexanders) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
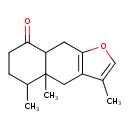
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H20O2 |
|---|
| Average Molecular Mass | 232.318 g/mol |
|---|
| Monoisotopic Mass | 232.146 g/mol |
|---|
| CAS Registry Number | 52061-43-5 |
|---|
| IUPAC Name | 3,4a,5-trimethyl-4H,4aH,5H,6H,7H,8H,8aH,9H-naphtho[2,3-b]furan-8-one |
|---|
| Traditional Name | 3,4a,5-trimethyl-4H,5H,6H,7H,8aH,9H-naphtho[2,3-b]furan-8-one |
|---|
| SMILES | CC1CCC(=O)C2CC3=C(CC12C)C(C)=CO3 |
|---|
| InChI Identifier | InChI=1S/C15H20O2/c1-9-8-17-14-6-12-13(16)5-4-10(2)15(12,3)7-11(9)14/h8,10,12H,4-7H2,1-3H3 |
|---|
| InChI Key | MHEQQQWHNMVBFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids. These are sesquiterpenoids with a structure based either on the eremophilane skeleton, its 8,9-seco derivative, or the furoeremophilane skeleton. Eremophilanes have been shown to be derived from eudesmanes by migration of the methyl group at C-10 to C-5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furoeremophilane sesquiterpenoid
- Naphthofuran
- Benzofuran
- Heteroaromatic compound
- Furan
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fb9-4940000000-ede8c606d6c11b74e691 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0190000000-6eff008932f1a136fbc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kur-3960000000-f19a2226b5d4c62b79c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktf-9200000000-9651be76f255a3000ce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-99d52d8d44d77d9978f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0190000000-9d51749163d3c8081ab1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f76-3940000000-8dfff351012cb3da6abe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-28de1d95e94e9d3723d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-2960000000-11ab27bf949512008dc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-7910000000-d8a88e53c34decfdc48a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-403b175a8d75af9b9dc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-403b175a8d75af9b9dc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004r-0970000000-729779e2bc7c5878e531 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036148 |
|---|
| FooDB ID | FDB014999 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00017374 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4359439 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5187560 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|