| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:31:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:58 UTC |
|---|
| Accession Number | CHEM029925 |
|---|
| Identification |
|---|
| Common Name | Limonexic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Citrus subspecies Limonexic acid is found in sweet orange and citrus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
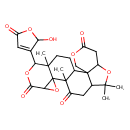
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Limonexate | Generator | | Limonexin | HMDB | | Shihulimonin a | HMDB |
|
|---|
| Chemical Formula | C26H30O10 |
|---|
| Average Molecular Mass | 502.510 g/mol |
|---|
| Monoisotopic Mass | 502.184 g/mol |
|---|
| CAS Registry Number | 99026-99-0 |
|---|
| IUPAC Name | 19-(2-hydroxy-5-oxo-2,5-dihydrofuran-3-yl)-9,9,13,20-tetramethyl-4,8,15,18-tetraoxahexacyclo[11.9.0.0²,⁷.0²,¹⁰.0¹⁴,¹⁶.0¹⁴,²⁰]docosane-5,12,17-trione |
|---|
| Traditional Name | 19-(2-hydroxy-5-oxo-2H-furan-3-yl)-9,9,13,20-tetramethyl-4,8,15,18-tetraoxahexacyclo[11.9.0.0²,⁷.0²,¹⁰.0¹⁴,¹⁶.0¹⁴,²⁰]docosane-5,12,17-trione |
|---|
| SMILES | CC1(C)OC2CC(=O)OCC22C1CC(=O)C1(C)C2CCC2(C)C(OC(=O)C3OC123)C1=CC(=O)OC1O |
|---|
| InChI Identifier | InChI=1S/C26H30O10/c1-22(2)13-8-14(27)24(4)12(25(13)10-32-16(28)9-15(25)35-22)5-6-23(3)18(11-7-17(29)33-20(11)30)34-21(31)19-26(23,24)36-19/h7,12-13,15,18-20,30H,5-6,8-10H2,1-4H3 |
|---|
| InChI Key | RTPPVNISJHFPFX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as limonoids. These are highly oxygenated, modified terpenoids with a prototypical structure either containing or derived from a precursor with a 4,4,8-trimethyl-17-furanylsteroid skeleton. All naturally occurring citrus limonoids contain a furan ring attached to the D-ring, at C-17, as well as oxygen containing functional groups at C-3, C-4, C-7, C-16 and C-17. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Limonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Limonoid skeleton
- Steroid lactone
- 11-oxosteroid
- Oxosteroid
- 2-oxosteroid
- Steroid
- Naphthopyran
- Naphthalene
- Tricarboxylic acid or derivatives
- 1,4-dioxepane
- Delta valerolactone
- Dioxepane
- Delta_valerolactone
- Oxane
- Pyran
- 2-furanone
- Tetrahydrofuran
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Dihydrofuran
- Carboxylic acid ester
- Lactone
- Ketone
- Hemiacetal
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fu-1043900000-70c2fe4a483f77ba0968 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-074l-6025090000-3025e08431ce60c8cbf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0001930000-e07bb1050cc713e2ca9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k9m-0002900000-077516246d5230a2cdcd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-4974100000-de61690f44d7bc867aa5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0000920000-5fc6cda5ce0caf479d5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1000900000-57e000a0358b6bd4335d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9202500000-6090d00423e79a2d65d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000910000-a2eb9909c1dff4907215 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0000970000-f9edc24e822a85346298 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zor-2000910000-0d32d29d379ae7d2a40e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000390000-50351e2e43c8a178f386 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zg0-0010950000-5f846ad51591f6faaf25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0h5g-0540910000-088c4d131510c4869042 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036075 |
|---|
| FooDB ID | FDB014907 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 76419898 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|