| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:29:28 UTC |
|---|
| Update Date | 2016-11-09 01:18:58 UTC |
|---|
| Accession Number | CHEM029888 |
|---|
| Identification |
|---|
| Common Name | 7alpha-1(10->19)-Abeo-7-acetoxyobacun-9(11)-ene |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of the fruits of a Citrus-Poncirus hybrid. 7alpha-1(10->19)-Abeo-7-acetoxyobacun-9(11)-ene is found in citrus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
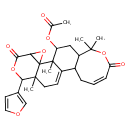
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7a-1(10->19)-abeo-7-acetoxyobacun-9(11)-ene | Generator | | 7Α-1(10->19)-abeo-7-acetoxyobacun-9(11)-ene | Generator | | (4Z)-18-(Furan-3-yl)-8,8,12,19-tetramethyl-6,16-dioxo-7,14,17-trioxapentacyclo[10.9.0.0²,⁹.0¹³,¹⁵.0¹³,¹⁹]henicosa-1(21),4-dien-11-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C28H32O8 |
|---|
| Average Molecular Mass | 496.549 g/mol |
|---|
| Monoisotopic Mass | 496.210 g/mol |
|---|
| CAS Registry Number | 85643-97-6 |
|---|
| IUPAC Name | (4Z)-18-(furan-3-yl)-8,8,12,19-tetramethyl-6,16-dioxo-7,14,17-trioxapentacyclo[10.9.0.0²,⁹.0¹³,¹⁵.0¹³,¹⁹]henicosa-1(21),4-dien-11-yl acetate |
|---|
| Traditional Name | (4Z)-18-(furan-3-yl)-8,8,12,19-tetramethyl-6,16-dioxo-7,14,17-trioxapentacyclo[10.9.0.0²,⁹.0¹³,¹⁵.0¹³,¹⁹]henicosa-1(21),4-dien-11-yl acetate |
|---|
| SMILES | CC(=O)OC1CC2C(C\C=C/C(=O)OC2(C)C)C2=CCC3(C)C(OC(=O)C4OC34C12C)C1=COC=C1 |
|---|
| InChI Identifier | InChI=1S/C28H32O8/c1-15(29)33-20-13-19-17(7-6-8-21(30)35-25(19,2)3)18-9-11-26(4)22(16-10-12-32-14-16)34-24(31)23-28(26,36-23)27(18,20)5/h6,8-10,12,14,17,19-20,22-23H,7,11,13H2,1-5H3/b8-6- |
|---|
| InChI Key | VIZDPIMTQWFZEF-VURMDHGXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0w3c-3022900000-80825eae52c841b8d31d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002k-0000900000-2d06e5728c5b1ef09515 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0570-0021900000-7eb66ccc2cf673de4d83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001d-9127200000-cbce69d9b40773bef734 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udj-0000900000-9b6b574592048176d0cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-2000900000-7d269d7578e413a6c8ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9002600000-13686c7e43b4a858f282 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-1a381b4ae37add237a32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0210900000-c62a46c38b53d8f9d304 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-0790400000-6ba854eba09e92fbde36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-b13e75a9e37a1aad48b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-323328ab38901ec47a98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-8000900000-e92a79e43bdfc495bad7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036033 |
|---|
| FooDB ID | FDB014852 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751902 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|