| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:26:53 UTC |
|---|
| Update Date | 2016-11-09 01:18:57 UTC |
|---|
| Accession Number | CHEM029829 |
|---|
| Identification |
|---|
| Common Name | (17alpha,23S)-17,23-Epoxy-29-hydroxy-27-norlanosta-1,8-diene-3,15,24-trione |
|---|
| Class | Small Molecule |
|---|
| Description | (17alpha,23S)-17,23-Epoxy-29-hydroxy-27-norlanosta-1,8-diene-3,15,24-trione is found in herbs and spices. (17alpha,23S)-17,23-Epoxy-29-hydroxy-27-norlanosta-1,8-diene-3,15,24-trione is a constituent of Muscari comosum (tassel hyacinth) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
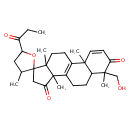
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (17a,23S)-17,23-Epoxy-29-hydroxy-27-norlanosta-1,8-diene-3,15,24-trione | Generator | | (17Α,23S)-17,23-epoxy-29-hydroxy-27-norlanosta-1,8-diene-3,15,24-trione | Generator | | 5b-1,3,7(11)-Eudesmatrien-8-one | HMDB | | 5Β-1,3,7(11)-eudesmatrien-8-one | HMDB |
|
|---|
| Chemical Formula | C29H40O5 |
|---|
| Average Molecular Mass | 468.625 g/mol |
|---|
| Monoisotopic Mass | 468.288 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 6'-(hydroxymethyl)-2',3,6',11',15'-pentamethyl-5-propanoylspiro[oxolane-2,14'-tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane]-1'(10'),3'-diene-5',12'-dione |
|---|
| Traditional Name | 6'-(hydroxymethyl)-2',3,6',11',15'-pentamethyl-5-propanoylspiro[oxolane-2,14'-tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane]-1'(10'),3'-diene-5',12'-dione |
|---|
| SMILES | CCC(=O)C1CC(C)C2(CC(=O)C3(C)C4=C(CCC23C)C2(C)C=CC(=O)C(C)(CO)C2CC4)O1 |
|---|
| InChI Identifier | InChI=1S/C29H40O5/c1-7-20(31)21-14-17(2)29(34-21)15-24(33)28(6)19-8-9-22-25(3,18(19)10-13-27(28,29)5)12-11-23(32)26(22,4)16-30/h11-12,17,21-22,30H,7-10,13-16H2,1-6H3 |
|---|
| InChI Key | BQWYLPVWSPZWPN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxosteroids. These are steroid derivatives carrying a C=O group attached to steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Oxosteroids |
|---|
| Direct Parent | Oxosteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-oxo-delta-1-steroid
- 3-oxosteroid
- 15-oxosteroid
- Oxosteroid
- Delta-1-steroid
- Cyclohexenone
- Tetrahydrofuran
- Cyclic ketone
- Ketone
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Oxacycle
- Primary alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05p9-4216900000-bd669b897a174019ac6c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a6s-6404960000-789b661f175dca70fb1e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0003900000-8add5a4d16f1e237920c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uyi-1155900000-67bfec72f6411fdb0571 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0197000000-4d78a1524512219e9dfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-21f08bdd9297c172ab9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05n0-1001900000-bef40b94b72fb72e727f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pyi-1019300000-cafbc5170cf85a3e2956 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-ad3c86b33d6cb3806a40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-3005900000-5e61770229f94e89d0db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9003300000-3bb24259216a9fe569c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0001900000-1a805061d024a5266b9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w29-0043900000-170e06892ac200816ce9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ar-8276900000-9596a70682c83b14ea57 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035971 |
|---|
| FooDB ID | FDB014775 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 74886443 |
|---|
| ChEBI ID | 175678 |
|---|
| PubChem Compound ID | 131751892 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|