| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:23:32 UTC |
|---|
| Update Date | 2016-11-09 01:18:56 UTC |
|---|
| Accession Number | CHEM029757 |
|---|
| Identification |
|---|
| Common Name | Grosheimin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
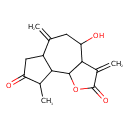
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H18O4 |
|---|
| Average Molecular Mass | 262.301 g/mol |
|---|
| Monoisotopic Mass | 262.121 g/mol |
|---|
| CAS Registry Number | 22489-66-3 |
|---|
| IUPAC Name | 4-hydroxy-9-methyl-3,6-dimethylidene-dodecahydroazuleno[4,5-b]furan-2,8-dione |
|---|
| Traditional Name | 4-hydroxy-9-methyl-3,6-dimethylidene-octahydroazuleno[4,5-b]furan-2,8-dione |
|---|
| SMILES | CC1C2C3OC(=O)C(=C)C3C(O)CC(=C)C2CC1=O |
|---|
| InChI Identifier | InChI=1S/C15H18O4/c1-6-4-11(17)13-8(3)15(18)19-14(13)12-7(2)10(16)5-9(6)12/h7,9,11-14,17H,1,3-5H2,2H3 |
|---|
| InChI Key | YGMIBVIKXJJQQJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as guaianolides and derivatives. These are diterpene lactones with a structure characterized by the presence of a gamma-lactone fused to a guaiane, forming 3,6,9-trimethyl-azuleno[4,5-b]furan-2-one or a derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Guaianolides and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Guaianolide-skeleton
- Guaiane sesquiterpenoid
- Sesquiterpenoid
- Gamma butyrolactone
- Cyclic alcohol
- Tetrahydrofuran
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Secondary alcohol
- Lactone
- Ketone
- Carboxylic acid ester
- Cyclic ketone
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0190000000-0540d20474782bf3fb62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vt-0690000000-b9b30483196b9ab2e09b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxu-9820000000-a17e83da187ce5911fba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-160857df66d82a2df5ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xu-0090000000-8c3cc5a92665e415c59e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uel-9620000000-140666a741e54aec42b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-da9d0d5c94a2c230a2b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0490000000-8e106798a130462c302d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rj-1970000000-d72fc2dd8211965429eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-9e603373aa78758870d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0290000000-d81b674584777c7372ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0096-1950000000-a38d73758241fb91bfe3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303491 |
|---|
| FooDB ID | FDB014671 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 90324 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 99967 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|