| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:20:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029681 |
|---|
| Identification |
|---|
| Common Name | S-Japonin |
|---|
| Class | Small Molecule |
|---|
| Description | S-Japonin is found in giant butterbur. S-Japonin is a constituent of leaves of Petasites japonicus |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
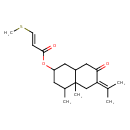
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,4a-Dimethyl-7-oxo-6-(propan-2-ylidene)-decahydronaphthalen-2-yl (2E)-3-(methylsulfanyl)prop-2-enoic acid | HMDB | | 4,4a-Dimethyl-7-oxo-6-(propan-2-ylidene)-decahydronaphthalen-2-yl (2E)-3-(methylsulphanyl)prop-2-enoate | HMDB | | 4,4a-Dimethyl-7-oxo-6-(propan-2-ylidene)-decahydronaphthalen-2-yl (2E)-3-(methylsulphanyl)prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C19H28O3S |
|---|
| Average Molecular Mass | 336.489 g/mol |
|---|
| Monoisotopic Mass | 336.176 g/mol |
|---|
| CAS Registry Number | 36031-35-3 |
|---|
| IUPAC Name | 4,4a-dimethyl-7-oxo-6-(propan-2-ylidene)-decahydronaphthalen-2-yl (2E)-3-(methylsulfanyl)prop-2-enoate |
|---|
| Traditional Name | 4,4a-dimethyl-7-oxo-6-(propan-2-ylidene)-hexahydro-1H-naphthalen-2-yl (2E)-3-(methylsulfanyl)prop-2-enoate |
|---|
| SMILES | CS\C=C\C(=O)OC1CC(C)C2(C)CC(=C(C)C)C(=O)CC2C1 |
|---|
| InChI Identifier | InChI=1S/C19H28O3S/c1-12(2)16-11-19(4)13(3)8-15(9-14(19)10-17(16)20)22-18(21)6-7-23-5/h6-7,13-15H,8-11H2,1-5H3/b7-6+ |
|---|
| InChI Key | HDHDUJDLKYTRAS-VOTSOKGWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids. These are sesquiterpenoids with a structure based either on the eremophilane skeleton, its 8,9-seco derivative, or the furoeremophilane skeleton. Eremophilanes have been shown to be derived from eudesmanes by migration of the methyl group at C-10 to C-5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Eremophilane sesquiterpenoid
- Vinylogous thioester
- Enoate ester
- Acrylic acid or derivatives
- Alpha,beta-unsaturated carboxylic ester
- Carboxylic acid ester
- Ketone
- Thioenolether
- Cyclic ketone
- Sulfenyl compound
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01xx-1952000000-40af6bcea89768b83031 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1479000000-030baf3c3b992c59730e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uy0-3981000000-3af7340135c15311e7a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-6920000000-60364e5c745446ed5db0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000j-9078000000-ebad5a52f791fee55e83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000b-9051000000-61d5583c2ee8cf203f47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9160000000-49b8592cbdb163268f6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0492000000-14710c2bc834e7d2ff1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-6091000000-c70b58cde11e5f8b7725 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014s-3090000000-71ee5f418ff35474a8a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0096000000-ef105ad82f143566821f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-3590000000-7192e904e387994154d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-6970000000-972c876047cf61b9e7ea | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035802 |
|---|
| FooDB ID | FDB014554 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00017015 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014024 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751866 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|