| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:19:47 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029672 |
|---|
| Identification |
|---|
| Common Name | (E)-4,8-Dimethyl-1,3,7-nonatriene |
|---|
| Class | Small Molecule |
|---|
| Description | An alkatriene consisting of 4,8-dimethylnonane having the three double bonds in the 1-, 3- and 7-positions. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
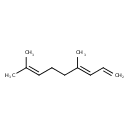
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3E)-4,8-Dimethylnona-1,3,7-triene | ChEBI | | (3E)-4,8-Dimethyl-1,3,7-nonatriene | HMDB | | (e)-4,8-Dimethylnona-1, 3, 7-triene | HMDB | | 4,8-Dimethyl-1,3(e),7-nonatriene | HMDB | | 3E-DMNT | MeSH, HMDB | | 4,8-Dimethyl-1,3,7-nonatriene | MeSH, HMDB |
|
|---|
| Chemical Formula | C11H18 |
|---|
| Average Molecular Mass | 150.261 g/mol |
|---|
| Monoisotopic Mass | 150.141 g/mol |
|---|
| CAS Registry Number | 19945-61-0 |
|---|
| IUPAC Name | (3E)-4,8-dimethylnona-1,3,7-triene |
|---|
| Traditional Name | (3E)-4,8-dimethylnona-1,3,7-triene |
|---|
| SMILES | CC(C)=CCC\C(C)=C\C=C |
|---|
| InChI Identifier | InChI=1S/C11H18/c1-5-7-11(4)9-6-8-10(2)3/h5,7-8H,1,6,9H2,2-4H3/b11-7+ |
|---|
| InChI Key | LUKZREJJLWEWQM-YRNVUSSQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic monoterpenoids. These are monoterpenes that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Acyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic monoterpenoid
- Branched unsaturated hydrocarbon
- Alkatriene
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Acyclic olefin
- Hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-9200000000-e416414d9be2f3adda39 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1900000000-33cc795ed1613576aebe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-1000-9800000000-3a1b3192b164c904567f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-091b7506f2aa97563857 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-a936ecc0d1be10048edb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-74b48f0ac52505674fca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o0-9800000000-f163ea5be1398af22c44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-6a71459d3b4a02d7d242 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-0900000000-55342dcd60eea7c1c043 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9400000000-0adddb098526474ebb96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-9100000000-21bc151a1aa29d67ee81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9000000000-6cee793406255179fd3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-7d936482d7d12f0e310d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035792 |
|---|
| FooDB ID | FDB014542 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00011383 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-8844 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4932528 |
|---|
| ChEBI ID | 60158 |
|---|
| PubChem Compound ID | 6427110 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|