| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:18:38 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029649 |
|---|
| Identification |
|---|
| Common Name | Piperalol |
|---|
| Class | Small Molecule |
|---|
| Description | Piperalol is found in mushrooms. Piperalol is a constituent of Lactarius piperatus |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
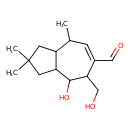
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8,14-Dihydroxy-4(6)-lactaren-5-al | HMDB |
|
|---|
| Chemical Formula | C15H24O3 |
|---|
| Average Molecular Mass | 252.349 g/mol |
|---|
| Monoisotopic Mass | 252.173 g/mol |
|---|
| CAS Registry Number | 100288-35-5 |
|---|
| IUPAC Name | 4-hydroxy-5-(hydroxymethyl)-2,2,8-trimethyl-1,2,3,3a,4,5,8,8a-octahydroazulene-6-carbaldehyde |
|---|
| Traditional Name | 4-hydroxy-5-(hydroxymethyl)-2,2,8-trimethyl-3,3a,4,5,8,8a-hexahydro-1H-azulene-6-carbaldehyde |
|---|
| SMILES | CC1C=C(C=O)C(CO)C(O)C2CC(C)(C)CC12 |
|---|
| InChI Identifier | InChI=1S/C15H24O3/c1-9-4-10(7-16)13(8-17)14(18)12-6-15(2,3)5-11(9)12/h4,7,9,11-14,17-18H,5-6,8H2,1-3H3 |
|---|
| InChI Key | MABZIKXHSLOMDZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as secondary alcohols. Secondary alcohols are compounds containing a secondary alcohol functional group, with the general structure HOC(R)(R') (R,R'=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Secondary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Carbonyl group
- Aldehyde
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-2890000000-d49b1c439184529c693d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001j-5419000000-393af733f5803c09c70c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0090000000-f93606793c6662735058 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0190000000-a44feb780437b6f16f60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0690-5950000000-fb3c1028e33fde8c0fa4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-8bf1880a56ce58295361 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-0090000000-0cf63f3958d8b4793e6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9650000000-5c4b04dd8a596bb43d4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0090000000-77eaa6ecd68a84c9d186 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-059i-3490000000-62cb7b975781e348839f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-9400000000-c26c834be2c70ab9ca26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ue9-0090000000-ac8f3f083a5cc04cb38a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-0090000000-fd41e12b60ddf8ae701c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0960000000-851939baf8be2282dec3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035767 |
|---|
| FooDB ID | FDB014505 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021551 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014015 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14412873 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|