| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:18:36 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029648 |
|---|
| Identification |
|---|
| Common Name | Ascaridole |
|---|
| Class | Small Molecule |
|---|
| Description | A p-menthane monoterpenoid that is p-menth-2-ene with a peroxy group across position 1 to 4. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
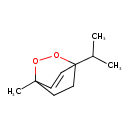
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Epidioxy-p-menth-2-ene | ChEBI | | 1,4-Peroxido-p-menthene-2 | ChEBI | | 1,4-Peroxy-p-menth-2-ene | ChEBI | | 1-Isopropyl-4-methyl-2,3-dioxabicyclo[2.2.2]oct-5-ene | ChEBI | | 1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo[2.2.2]oct-5-ene | ChEBI | | 1, 4-Epidioxy-P-menth-2-ene | HMDB | | 1, 4-Peroxy-P-menth-2-ene | HMDB | | 1,4-Epidioxy-2-P-menthene | HMDB | | 1-Isopropyl-4-methyl-2,3-dioxabicyclo(2.2.2)oct-5-ene | HMDB | | 1-Isopropyl-4-methyl-7-oxabicyclo[2.2.1]hept-2-ene | HMDB | | 1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo(2.2.2)oct-5-ene | HMDB | | 1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo[2.2.2]oct-5-ene, 9ci | HMDB | | 4-Methyl-1-(propan-2-yl)-2,3-dioxabicyclo[2.2.2]oct-5-ene | HMDB | | Ascapurin | HMDB | | Ascaricum | HMDB | | Ascaridiol | HMDB | | Ascaridol | HMDB | | Ascaridole (organic peroxide) | HMDB | | Ascaridole epoxide | HMDB | | Ascarisin | HMDB | | Askaridol | HMDB | | cis-Ascaridole | HMDB | | Kebal II | HMDB | | Uncinacina | HMDB |
|
|---|
| Chemical Formula | C10H16O2 |
|---|
| Average Molecular Mass | 168.233 g/mol |
|---|
| Monoisotopic Mass | 168.115 g/mol |
|---|
| CAS Registry Number | 512-85-6 |
|---|
| IUPAC Name | 1-methyl-4-(propan-2-yl)-2,3-dioxabicyclo[2.2.2]oct-5-ene |
|---|
| Traditional Name | ascaridole |
|---|
| SMILES | CC(C)C12CCC(C)(OO1)C=C2 |
|---|
| InChI Identifier | InChI=1S/C10H16O2/c1-8(2)10-6-4-9(3,5-7-10)11-12-10/h4,6,8H,5,7H2,1-3H3 |
|---|
| InChI Key | MGYMHQJELJYRQS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-dioxanes. These are organic compounds containing 1,2-dioxane, an aliphatic six-member ring with two oxygen atoms in ring positions 1 and 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxanes |
|---|
| Sub Class | 1,2-dioxanes |
|---|
| Direct Parent | 1,2-dioxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ortho-dioxane
- Dialkyl peroxide
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2900000000-4e6342ba9b29d1dd42ad | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-83d7b2bd24b624b5ea98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-1d0d1c7edaaa69d194a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0900000000-2c78632dd190511927b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-177dc272267c0f543701 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-3317598e1aa469191e58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adi-0900000000-fb70c59640ba1c0d01a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-cb7592114e600c180aec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-cb7592114e600c180aec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-3a7e13f32c837bc356ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-f9b2951dd69b378c6031 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0900000000-460c95031436dfd1e1b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0900000000-6a28e536e83eb1686dba | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035766 |
|---|
| FooDB ID | FDB014503 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003027 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ascaridole |
|---|
| Chemspider ID | 10105 |
|---|
| ChEBI ID | 2866 |
|---|
| PubChem Compound ID | 10545 |
|---|
| Kegg Compound ID | C09836 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|