| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:18:11 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029639 |
|---|
| Identification |
|---|
| Common Name | Phytuberin |
|---|
| Class | Small Molecule |
|---|
| Description | Phytoalexin of potato tubers infected with Erwinia carotovora. Phytuberin is found in potato. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
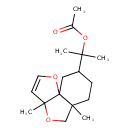
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Phytuberin | HMDB | | (3AR,5as,8R,9ar)-5,5a,6,7,8,9-hexahydro-a,a,3a,5a-tetramethyl-3ah-furo[3,2-c]isobenzofuran-8-methanol acetate | HMDB | | 2-{5,8-dimethyl-2,6-dioxatricyclo[6.4.0.0¹,⁵]dodec-3-en-11-yl}propan-2-yl acetic acid | Generator | | Phytuberin | MeSH |
|
|---|
| Chemical Formula | C17H26O4 |
|---|
| Average Molecular Mass | 294.386 g/mol |
|---|
| Monoisotopic Mass | 294.183 g/mol |
|---|
| CAS Registry Number | 37209-50-0 |
|---|
| IUPAC Name | 2-{5,8-dimethyl-2,6-dioxatricyclo[6.4.0.0¹,⁵]dodec-3-en-11-yl}propan-2-yl acetate |
|---|
| Traditional Name | 2-{5,8-dimethyl-2,6-dioxatricyclo[6.4.0.0¹,⁵]dodec-3-en-11-yl}propan-2-yl acetate |
|---|
| SMILES | CC(=O)OC(C)(C)C1CCC2(C)COC3(C)C=COC23C1 |
|---|
| InChI Identifier | InChI=1S/C17H26O4/c1-12(18)21-14(2,3)13-6-7-15(4)11-20-16(5)8-9-19-17(15,16)10-13/h8-9,13H,6-7,10-11H2,1-5H3 |
|---|
| InChI Key | YARAJYKHRCCDLG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Furofuran
- Dihydrofuran
- Tetrahydrofuran
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-8790000000-204f18f03337448806bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-cc76c763cbe31dfdd96d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7a-0290000000-5b04d6d01ecfb38f4027 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9l-9470000000-c2b72c5c1de2ed990b8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1090000000-82afbf69bdcfb43dd30a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udl-2090000000-97ea2256b67bbbd6f922 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-3090000000-a9065826352da11fcf26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-5f8ee6859cac66bfcaaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-3690000000-28de909c0b3cd35a9d2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdu-9720000000-b5aa3acb717923280f3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-46f655010b6b64c43b61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-6090000000-8fce51c00773e97d8713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0c2c-2090000000-e2c6f8948b470842d2a3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035754 |
|---|
| FooDB ID | FDB014488 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003172 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 278792 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 315114 |
|---|
| Kegg Compound ID | C09709 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|