| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:17:29 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029622 |
|---|
| Identification |
|---|
| Common Name | Dukunolide E |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Lansium domesticum (langsat). Dukunolide E is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
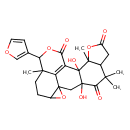
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H28O9 |
|---|
| Average Molecular Mass | 484.495 g/mol |
|---|
| Monoisotopic Mass | 484.173 g/mol |
|---|
| CAS Registry Number | 101559-97-1 |
|---|
| IUPAC Name | 15-(furan-3-yl)-3,11-dihydroxy-5,5,10,16-tetramethyl-9,14,20-trioxahexacyclo[10.8.1.0¹,¹⁹.0³,¹¹.0⁶,¹⁰.0¹⁶,²¹]henicos-12(21)-ene-4,8,13-trione |
|---|
| Traditional Name | 15-(furan-3-yl)-3,11-dihydroxy-5,5,10,16-tetramethyl-9,14,20-trioxahexacyclo[10.8.1.0¹,¹⁹.0³,¹¹.0⁶,¹⁰.0¹⁶,²¹]henicos-12(21)-ene-4,8,13-trione |
|---|
| SMILES | CC12OC(=O)CC1C(C)(C)C(=O)C1(O)CC34OC3CCC3(C)C(OC(=O)C(=C43)C21O)C1=COC=C1 |
|---|
| InChI Identifier | InChI=1S/C26H28O9/c1-21(2)13-9-15(27)35-23(13,4)26(31)16-17-22(3,18(33-19(16)28)12-6-8-32-10-12)7-5-14-24(17,34-14)11-25(26,30)20(21)29/h6,8,10,13-14,18,30-31H,5,7,9,11H2,1-4H3 |
|---|
| InChI Key | HQKQACOUFFEUAD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05mx-9223700000-a889ef565f006797c75f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0mu3-9200062000-0d0921184589cf733610 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Dukunolide E,1TMS,#2" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0000900000-5ded0918c768b6d868a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-0010900000-85a46537356002f14d7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kk-9601100000-11edfb0d386ccfb1fb74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-0000900000-8774d304fcbc26e13aa5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00li-1000900000-6a0082b111aaedc14b7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9403600000-11880d08dd94071981e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-b0baef7886ad1d902928 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000900000-a21a7e24214c052b6bf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-1010-0020900000-47b000e187bf511a7197 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-5e6c5052e0b683e8326f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-0000900000-f6e1551d5b7ecc2a6c69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000e-7180900000-ce52ad2f8946b536157b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035733 |
|---|
| FooDB ID | FDB014461 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751857 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|