| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:17:27 UTC |
|---|
| Update Date | 2016-11-09 01:18:55 UTC |
|---|
| Accession Number | CHEM029621 |
|---|
| Identification |
|---|
| Common Name | Dukunolide D |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Lansium domesticum (langsat). Dukunolide D is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
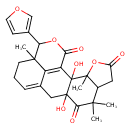
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Dukunolide D | HMDB | | 1,1'-Dimethyl-2,2'-oxydiethyl dibenzoate | HMDB | | Dipropylene glycol dibenzoate | HMDB | | Oxydipropane-1,2-diyl dibenzoate | HMDB | | Propanol, oxybis-, dibenzoate | HMDB |
|
|---|
| Chemical Formula | C26H28O8 |
|---|
| Average Molecular Mass | 468.496 g/mol |
|---|
| Monoisotopic Mass | 468.178 g/mol |
|---|
| CAS Registry Number | 101560-02-5 |
|---|
| IUPAC Name | 17-(furan-3-yl)-2,10-dihydroxy-3,8,8,16-tetramethyl-4,18-dioxapentacyclo[10.7.1.0²,¹⁰.0³,⁷.0¹⁶,²⁰]icosa-1(20),12-diene-5,9,19-trione |
|---|
| Traditional Name | 17-(furan-3-yl)-2,10-dihydroxy-3,8,8,16-tetramethyl-4,18-dioxapentacyclo[10.7.1.0²,¹⁰.0³,⁷.0¹⁶,²⁰]icosa-1(20),12-diene-5,9,19-trione |
|---|
| SMILES | CC12OC(=O)CC1C(C)(C)C(=O)C1(O)CC3=CCCC4(C)C(OC(=O)C(=C34)C21O)C1=COC=C1 |
|---|
| InChI Identifier | InChI=1S/C26H28O8/c1-22(2)15-10-16(27)34-24(15,4)26(31)18-17-13(11-25(26,30)21(22)29)6-5-8-23(17,3)19(33-20(18)28)14-7-9-32-12-14/h6-7,9,12,15,19,30-31H,5,8,10-11H2,1-4H3 |
|---|
| InChI Key | WUQGZJQZKDLECQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthopyran
- Naphthofuran
- Naphthalene
- Dihydropyranone
- Dicarboxylic acid or derivatives
- Gamma butyrolactone
- Pyran
- Cyclic alcohol
- Furan
- Heteroaromatic compound
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Tertiary alcohol
- Tetrahydrofuran
- Lactone
- Ketone
- Carboxylic acid ester
- 1,2-diol
- Oxacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-8265900000-c34c57fb27d3982e3758 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0gi4-9210080000-5ea1e8a9def7bfc53f75 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Dukunolide D,1TMS,#2" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-05fe7f8d93d043bf3433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0000900000-6fdaa93c85af22bc5e1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kk-9501000000-2a02a2ad319d0a5f3dd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-0000900000-8b9d04d5395dfda91981 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01b9-1001900000-8eeebfd2f38b366c0b46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9406200000-9bbd100d16ee7179b3c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-cc33c2bebaeb72d73a58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gdi-0100900000-8a3998899426dbf3542a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-5491400000-57a12129d86a786ec452 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-7dccc54e01e7cdbb03b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000900000-9e5d2ae0d9d409a74039 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01dj-7079600000-fbb87fc0e88f252b9485 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035732 |
|---|
| FooDB ID | FDB014460 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00035090 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13970419 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|