| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:16:20 UTC |
|---|
| Update Date | 2016-11-09 01:18:54 UTC |
|---|
| Accession Number | CHEM029597 |
|---|
| Identification |
|---|
| Common Name | (6alpha,7alpha,10alpha)-1(5),3-Aromadendradiene |
|---|
| Class | Small Molecule |
|---|
| Description | (6alpha,7alpha,10alpha)-1(5),3-Aromadendradiene is a constituent of Tolu balsam (Myroxylon balsamum var. balsamum). (6alpha,7alpha,10alpha)-1(5),3-Aromadendradiene is a flavouring agent |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
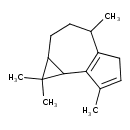
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6a,7a,10a)-1(5),3-Aromadendradiene | Generator | | (6Α,7α,10α)-1(5),3-aromadendradiene | Generator | | 1(5),3-Aromadendradiene | HMDB |
|
|---|
| Chemical Formula | C15H22 |
|---|
| Average Molecular Mass | 202.335 g/mol |
|---|
| Monoisotopic Mass | 202.172 g/mol |
|---|
| CAS Registry Number | 111778-06-4 |
|---|
| IUPAC Name | 1,1,4,7-tetramethyl-1H,1aH,2H,3H,4H,5H,7bH-cyclopropa[e]azulene |
|---|
| Traditional Name | 1,1,4,7-tetramethyl-1aH,2H,3H,4H,5H,7bH-cyclopropa[e]azulene |
|---|
| SMILES | CC1CCC2C(C3=C1CC=C3C)C2(C)C |
|---|
| InChI Identifier | InChI=1S/C15H22/c1-9-6-8-12-14(15(12,3)4)13-10(2)5-7-11(9)13/h5,9,12,14H,6-8H2,1-4H3 |
|---|
| InChI Key | YBLUUFTVJWBMRJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as azulenes. These are polycyclic aromatic compounds containing the azulene skeleton, which consists of the cyclopentadiene ring fused to a cycloheptadiene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Olefins |
|---|
| Direct Parent | Azulenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Azulene
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Unsaturated aliphatic hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0cdr-2900000000-d3ea10d9b23512232ae4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0290000000-e7663baaa99aa4c7857c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2980000000-d83ec2ef324f3fbf001b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-8900000000-9247b3ca5d7c7844ad17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-89acc32263f896870bc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-3a6ae6a68430fd244fe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-3900000000-aaba5d70d30dc2db8ddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-0b1ac00c3b3c6c0a73b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-0b1ac00c3b3c6c0a73b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0900000000-aec6ff6c96b0b2116295 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1190000000-ba8f2068071b3fdc4a12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pdi-6930000000-83971ec513f95aeca94c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056u-9800000000-a506336a4554ca561845 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035707 |
|---|
| FooDB ID | FDB014429 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021203 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013999 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13892028 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|