| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:15:44 UTC |
|---|
| Update Date | 2016-11-09 01:18:54 UTC |
|---|
| Accession Number | CHEM029584 |
|---|
| Identification |
|---|
| Common Name | 8,15-Isopimaradien-18-oic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 8,15-Isopimaradien-18-oic acid is found in resin of Pinus species especially Pinus edulis (pinon |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
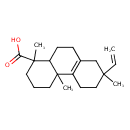
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8,15-Isopimaradien-18-Oate | Generator | | (4-Aminophenyl)-phosphonous acid | HMDB | | (P-Aminophenyl)-phosphonous acid | HMDB | | D8-Isopimaric acid | HMDB | | P-Aminobenzenephosphonous acid | HMDB | | 7-Ethenyl-1,4a,7-trimethyl-1,2,3,4,4a,5,6,7,8,9,10,10a-dodecahydrophenanthrene-1-carboxylate | Generator |
|
|---|
| Chemical Formula | C20H30O2 |
|---|
| Average Molecular Mass | 302.451 g/mol |
|---|
| Monoisotopic Mass | 302.225 g/mol |
|---|
| CAS Registry Number | 3625-01-2 |
|---|
| IUPAC Name | 7-ethenyl-1,4a,7-trimethyl-1,2,3,4,4a,5,6,7,8,9,10,10a-dodecahydrophenanthrene-1-carboxylic acid |
|---|
| Traditional Name | 7-ethenyl-1,4a,7-trimethyl-3,4,5,6,8,9,10,10a-octahydro-2H-phenanthrene-1-carboxylic acid |
|---|
| SMILES | CC1(CCC2=C(CCC3C(C)(CCCC23C)C(O)=O)C1)C=C |
|---|
| InChI Identifier | InChI=1S/C20H30O2/c1-5-18(2)12-9-15-14(13-18)7-8-16-19(15,3)10-6-11-20(16,4)17(21)22/h5,16H,1,6-13H2,2-4H3,(H,21,22) |
|---|
| InChI Key | BFPAVSSBPLQXJZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pimarane diterpenoid
- Diterpenoid
- Phenanthrene
- Hydrophenanthrene
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-0390000000-19197933fa908285ed42 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4r-5359000000-f4639f0013007bb5c7a9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0094000000-7e4daabbf2138964d365 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-3291000000-c30806ac23cf7c305703 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002r-5970000000-b24512ccbb58a4e4dac2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0059000000-a920f07c26cca434c8ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-0094000000-09643b2bc3799761342d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052o-1090000000-c1ad48c404e71eb1b7f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-16d58525184e918518fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0029000000-47ed0c138397e272fdfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udm-2093000000-97b69ec351796593cbda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zi0-0093000000-d5cecab97b12cf225c50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ufr-1792000000-689dcb50881b7a96fbe3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014m-5920000000-8eaee74b645b65661db5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035692 |
|---|
| FooDB ID | FDB014410 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056288 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 18730597 |
|---|
| ChEBI ID | 170106 |
|---|
| PubChem Compound ID | 15627707 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|