| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:15:32 UTC |
|---|
| Update Date | 2016-11-09 01:18:54 UTC |
|---|
| Accession Number | CHEM029580 |
|---|
| Identification |
|---|
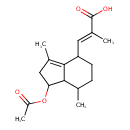
| Common Name | Acetylvalerenolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Acetylvalerenolic acid is found in fats and oils. Acetylvalerenolic acid is a constituent of Valeriana officinalis (valerian) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acetylvalerenolate | Generator | | Acetoxyvalerenic acid | HMDB | | (2E)-3-[1-(Acetyloxy)-3,7-dimethyl-2,4,5,6,7,7a-hexahydro-1H-inden-4-yl]-2-methylprop-2-enoate | Generator | | Acetylvalerenolic acid | MeSH |
|
|---|
| Chemical Formula | C17H24O4 |
|---|
| Average Molecular Mass | 292.370 g/mol |
|---|
| Monoisotopic Mass | 292.167 g/mol |
|---|
| CAS Registry Number | 81397-67-3 |
|---|
| IUPAC Name | (2E)-3-[1-(acetyloxy)-3,7-dimethyl-2,4,5,6,7,7a-hexahydro-1H-inden-4-yl]-2-methylprop-2-enoic acid |
|---|
| Traditional Name | (2E)-3-[1-(acetyloxy)-3,7-dimethyl-2,4,5,6,7,7a-hexahydro-1H-inden-4-yl]-2-methylprop-2-enoic acid |
|---|
| SMILES | CC1CCC(\C=C(/C)C(O)=O)C2=C(C)CC(OC(C)=O)C12 |
|---|
| InChI Identifier | InChI=1S/C17H24O4/c1-9-5-6-13(7-11(3)17(19)20)15-10(2)8-14(16(9)15)21-12(4)18/h7,9,13-14,16H,5-6,8H2,1-4H3,(H,19,20)/b11-7+ |
|---|
| InChI Key | VBBXZFLAYWAXSK-YRNVUSSQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6r-2290000000-529a7f8ec9eae9d4f258 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-056v-4196000000-21f2051cec69d0f86921 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-ed8845fdd32ab1b8dc33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uea-0490000000-4f820e61df23116ab3b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0khj-2920000000-0cfec4ff6c6899bdf241 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-1090000000-6506f1685409a1363043 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052e-2090000000-dd280e0d094f852bc6a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-7090000000-e2829d1e4755d594246a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a5i-8790000000-75cd8a17a975e1c42429 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-c01bbbf5bed889264ddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9630000000-90e3cd7f496fc0b00dab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0490000000-22ba0f07b19460e16e4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-0930000000-2d7ce207c462e83c0815 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9610000000-3514d958a46f27deb603 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035687 |
|---|
| FooDB ID | FDB014404 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021292 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4943984 |
|---|
| ChEBI ID | 172142 |
|---|
| PubChem Compound ID | 6439585 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|