| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:14:54 UTC |
|---|
| Update Date | 2016-11-09 01:18:54 UTC |
|---|
| Accession Number | CHEM029560 |
|---|
| Identification |
|---|
| Common Name | L-1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of beta-carbolines that is 1,2,3,4-tetrahydro-beta-carboline substituted at position 3 by a carboxy group. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
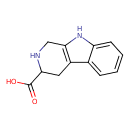
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Carboxy-1,2,3,4-tetrahydro-2-carboline | ChEBI | | CCRIS 6486 | ChEBI | | NSC 96912 | ChEBI | | L-1,2,3,4-Tetrahydro-b-carboline-3-carboxylate | Generator | | L-1,2,3,4-Tetrahydro-b-carboline-3-carboxylic acid | Generator | | L-1,2,3,4-Tetrahydro-beta-carboline-3-carboxylate | Generator | | L-1,2,3,4-Tetrahydro-β-carboline-3-carboxylate | Generator | | L-1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid | Generator | | (-)-3-Carboxy-1,2,3,4-tetrahydro-beta-carboline | HMDB | | 3-Carboxy-1,2,3,4-tetrahydro-beta-carboline | HMDB | | Cyclomethyltryptophan | HMDB | | L-1,2,3,4-Tetrahydronorharman-3-carboxylic acid | HMDB | | 1,2,3,4-Tetrahydro-b-carboline-3-carboxylate | Generator | | 1,2,3,4-Tetrahydro-b-carboline-3-carboxylic acid | Generator | | 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylate | Generator | | 1,2,3,4-Tetrahydro-β-carboline-3-carboxylate | Generator | | 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid | Generator | | 3S-Tetrahydro-beta-carboline-3-carboxylic acid | MeSH | | 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid, (S)-isomer | MeSH | | 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid,(+-)-isomer | MeSH | | THBC-CA | MeSH | | 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid | MeSH |
|
|---|
| Chemical Formula | C12H12N2O2 |
|---|
| Average Molecular Mass | 216.236 g/mol |
|---|
| Monoisotopic Mass | 216.090 g/mol |
|---|
| CAS Registry Number | 42438-90-4 |
|---|
| IUPAC Name | 1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylic acid |
|---|
| Traditional Name | 1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylic acid |
|---|
| SMILES | OC(=O)C1CC2=C(CN1)NC1=CC=CC=C21 |
|---|
| InChI Identifier | InChI=1S/C12H12N2O2/c15-12(16)10-5-8-7-3-1-2-4-9(7)14-11(8)6-13-10/h1-4,10,13-14H,5-6H2,(H,15,16) |
|---|
| InChI Key | FSNCEEGOMTYXKY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta carbolines. Beta carbolines are compounds containing a 9H-pyrido[3,4-b]indole moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Pyridoindoles |
|---|
| Direct Parent | Beta carbolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-carboline
- Alpha-amino acid
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Aralkylamine
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Amino acid
- Amino acid or derivatives
- Carboxylic acid derivative
- Secondary amine
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0900000000-e9244ddaab935e320f50 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-4910000000-0adb6a521b7d73eedf39 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-00r6-1920000000-dda4400f9395de4f47b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0690000000-e97754f079c7a0ca47ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0910000000-d5b709d072efe4806705 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-0900000000-91bea12068ce0b420576 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0390000000-94bcbc6157d022a380a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01b9-0960000000-386d3e65862e6bb7a5a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0993-1900000000-4456de60636dfdef613a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0190000000-118c13a6bdea90374741 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0910000000-054a6eb83d0d5f279434 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-f9dceeae7b086c1cd5b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0490000000-6feffc7378ee0b9763da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0960000000-84232f8202f3a396325d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009x-0900000000-76cc45db144566742285 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035665 |
|---|
| FooDB ID | FDB014381 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00026660 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 88749 |
|---|
| ChEBI ID | 91151 |
|---|
| PubChem Compound ID | 98285 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|