| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:12:13 UTC |
|---|
| Update Date | 2016-11-09 01:18:53 UTC |
|---|
| Accession Number | CHEM029500 |
|---|
| Identification |
|---|
| Common Name | Kahweol |
|---|
| Class | Small Molecule |
|---|
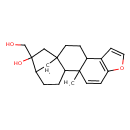
| Description | A diterpenoid with formula C20H26O3, isolated from the beans of Coffea arabica. It exhibits antioxidant, anti-inflammatory, anti-angiogenesis and anti-proliferative properties. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CCRIS 1521 | ChEBI | | CCRIS-1521 | ChEBI |
|
|---|
| Chemical Formula | C20H26O3 |
|---|
| Average Molecular Mass | 314.419 g/mol |
|---|
| Monoisotopic Mass | 314.188 g/mol |
|---|
| CAS Registry Number | 6894-43-5 |
|---|
| IUPAC Name | 17-(hydroxymethyl)-12-methyl-8-oxapentacyclo[14.2.1.0¹,¹³.0⁴,¹².0⁵,⁹]nonadeca-5(9),6,10-trien-17-ol |
|---|
| Traditional Name | 17-(hydroxymethyl)-12-methyl-8-oxapentacyclo[14.2.1.0¹,¹³.0⁴,¹².0⁵,⁹]nonadeca-5(9),6,10-trien-17-ol |
|---|
| SMILES | CC12C=CC3=C(C=CO3)C1CCC13CC(CCC21)C(O)(CO)C3 |
|---|
| InChI Identifier | InChI=1S/C20H26O3/c1-18-7-5-16-14(6-9-23-16)15(18)4-8-19-10-13(2-3-17(18)19)20(22,11-19)12-21/h5-7,9,13,15,17,21-22H,2-4,8,10-12H2,1H3 |
|---|
| InChI Key | JEKMKNDURXDJAD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthofuran
- Heteroaromatic compound
- Tertiary alcohol
- Furan
- Cyclic alcohol
- 1,2-diol
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000m-0590000000-cbf3c432ffc87aed9e80 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-002f-9534800000-58b55eccff30839320c3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0149000000-db23928410381e9a18f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0392000000-f4b88a6891473473ddf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0frw-5970000000-c8c248c27bb4efc9fdc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0039000000-61fb732de24a5e6c886a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0094000000-1c828fa69ffecae29c5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-1090000000-4bc978ebbf2a0ccf815c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-d8638daf32922c227464 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0019000000-f86639b6d0fdd901510d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-0093000000-15f5c908f4472d8d4215 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-88e0e67fa31725f576fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-3194000000-bb658b1aa87085b0e9e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001r-2930000000-0ebed8f5e58f9f6f24a4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00053387 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-16468 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Kahweol |
|---|
| Chemspider ID | 102755 |
|---|
| ChEBI ID | 138308 |
|---|
| PubChem Compound ID | 114778 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|