| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:10:06 UTC |
|---|
| Update Date | 2016-11-09 01:18:52 UTC |
|---|
| Accession Number | CHEM029429 |
|---|
| Identification |
|---|
| Common Name | Capsicum annuum Fluorescent chlorophyll catabolite |
|---|
| Class | Small Molecule |
|---|
| Description | Brassica napus fluorescent chlorophyll catabolite is found in brassicas. Chlorophyll catabolite isolated from Brassica napu |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
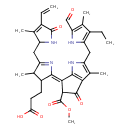
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ca-FCC-2 | HMDB | | 3-{5-[(4-ethenyl-5-hydroxy-3-methyl-2H-pyrrol-2-yl)methyl]-2-[(6Z)-2-[(3-ethyl-5-formyl-4-methyl-1H-pyrrol-2-yl)methyl]-5-(methoxycarbonyl)-3-methyl-4-oxo-1H,4H,5H,6H-cyclopenta[b]pyrrol-6-ylidene]-4-methyl-3,4-dihydro-2H-pyrrol-3-yl}propanoate | Generator |
|
|---|
| Chemical Formula | C35H40N4O7 |
|---|
| Average Molecular Mass | 628.715 g/mol |
|---|
| Monoisotopic Mass | 628.290 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-{5-[(4-ethenyl-3-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-yl)methyl]-2-[(6Z)-2-[(3-ethyl-5-formyl-4-methyl-1H-pyrrol-2-yl)methyl]-5-(methoxycarbonyl)-3-methyl-4-oxo-1H,4H,5H,6H-cyclopenta[b]pyrrol-6-ylidene]-4-methyl-3,4-dihydro-2H-pyrrol-3-yl}propanoic acid |
|---|
| Traditional Name | 3-{5-[(4-ethenyl-3-methyl-5-oxo-1,2-dihydropyrrol-2-yl)methyl]-2-[(6Z)-2-[(3-ethyl-5-formyl-4-methyl-1H-pyrrol-2-yl)methyl]-5-(methoxycarbonyl)-3-methyl-4-oxo-1H,5H-cyclopenta[b]pyrrol-6-ylidene]-4-methyl-3,4-dihydropyrrol-3-yl}propanoic acid |
|---|
| SMILES | CCC1=C(CC2=C(C)C3=C(N2)\C(C(C(=O)OC)C3=O)=C2/N=C(CC3NC(=O)C(C=C)=C3C)C(C)C2CCC(O)=O)NC(C=O)=C1C |
|---|
| InChI Identifier | InChI=1S/C35H40N4O7/c1-8-19-15(3)26(14-40)36-25(19)13-24-18(6)28-32(38-24)29(30(33(28)43)35(45)46-7)31-21(10-11-27(41)42)17(5)22(37-31)12-23-16(4)20(9-2)34(44)39-23/h9,14,17,21,23,30,36,38H,2,8,10-13H2,1,3-7H3,(H,39,44)(H,41,42)/b31-29- |
|---|
| InChI Key | ULSSSZOYSMVFIJ-YCNYHXFESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrapyrroles and derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrapyrroles and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrapyrrole skeleton
- Aryl ketone
- Aryl alkyl ketone
- Aryl-aldehyde
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- 1,3-dicarbonyl compound
- Vinylogous amide
- Heteroaromatic compound
- Methyl ester
- Pyrroline
- Pyrrole
- Carboxamide group
- Carboxylic acid ester
- Secondary carboxylic acid amide
- Ketimine
- Ketone
- Lactam
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Imine
- Aldehyde
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01w3-4550495000-c78649fb3191c1b85e49 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Capsicum annuum Fluorescent chlorophyll catabolite,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0000098000-cc5add653dff80deb73f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0hh0-0202093000-985fda345a42230e0fc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-7722190000-7d18436a01693cfb8161 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1010029000-2e9aa60e06c2e5341189 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056u-3204189000-beb9cb2fdf662f66440c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9100131000-0853cc46e02ee2bb561f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000439000-4d03958bd71f9d0f313d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02dl-0220597000-75ed193a2dbf3c47519c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015a-0100392000-17cf32efcd43aec10928 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000009000-a99e2139929897f3b4a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00c0-1101944000-799bf5b1e16369c47c32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00tp-3170393000-a7feabd4998c0775e759 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035528 |
|---|
| FooDB ID | FDB014220 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013949 |
|---|
| ChEBI ID | 168862 |
|---|
| PubChem Compound ID | 15330925 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|