| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:09:47 UTC |
|---|
| Update Date | 2016-11-09 01:18:52 UTC |
|---|
| Accession Number | CHEM029423 |
|---|
| Identification |
|---|
| Common Name | Ginsenoyne M |
|---|
| Class | Small Molecule |
|---|
| Description | Ginsenoyne M is found in tea. Ginsenoyne M is a constituent of the roots of Panax ginseng (ginseng) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
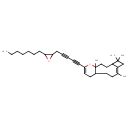
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C32H46O2 |
|---|
| Average Molecular Mass | 462.706 g/mol |
|---|
| Monoisotopic Mass | 462.350 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (7Z)-14-[5-(3-heptyloxiran-2-yl)penta-1,3-diyn-1-yl]-1,5,5,8-tetramethyl-15-oxatricyclo[9.4.0.0⁴,⁷]pentadeca-7,13-diene |
|---|
| Traditional Name | (7Z)-14-[5-(3-heptyloxiran-2-yl)penta-1,3-diyn-1-yl]-1,5,5,8-tetramethyl-15-oxatricyclo[9.4.0.0⁴,⁷]pentadeca-7,13-diene |
|---|
| SMILES | CCCCCCCC1OC1CC#CC#CC1=CCC2CC\C(C)=C3\CC(C)(C)C3CCC2(C)O1 |
|---|
| InChI Identifier | InChI=1S/C32H46O2/c1-6-7-8-9-12-15-29-30(33-29)16-13-10-11-14-26-20-19-25-18-17-24(2)27-23-31(3,4)28(27)21-22-32(25,5)34-26/h20,25,28-30H,6-9,12,15-19,21-23H2,1-5H3/b27-24- |
|---|
| InChI Key | UZINSQVBXBQDEQ-PNHLSOANSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as epoxides. Epoxides are compounds containing a cyclic ether with three ring atoms(one oxygen and two carbon atoms). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Epoxides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Epoxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxacycle
- Ether
- Oxirane
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-6223900000-775413d0e4d999312280 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1122900000-8b47e579c79ccd40737a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001a-7696200000-0daf22cea87a965c796b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ka6-9580000000-3defae1867d0ef1f1d6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0111900000-53050895c8a956baa726 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0983700000-b3c96114a14cbd22389a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8953300000-b9c2f997e816eb47c2ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-a09d75024f62f6c4e97a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0103900000-0fe9b6caf72bd004e31b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-0009200000-809553bd4cd96ed37775 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0001900000-0b293e24536b61cf0907 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1014900000-8d50426d8925d277b51c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abc-7019100000-9285b5490bfa7567bf68 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035522 |
|---|
| FooDB ID | FDB014214 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013945 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10595178 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|