| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:08:59 UTC |
|---|
| Update Date | 2016-11-09 01:18:52 UTC |
|---|
| Accession Number | CHEM029405 |
|---|
| Identification |
|---|
| Common Name | Epicatechin-(4beta->6)-epicatechin-(2beta->7,4beta->8)-epicatechin |
|---|
| Class | Small Molecule |
|---|
| Description | Epicatechin-(4beta->6)-epicatechin-(2beta->7,4beta->8)-epicatechin is found in fruits. Epicatechin-(4beta->6)-epicatechin-(2beta->7,4beta->8)-epicatechin is a constituent of the fruit of Vaccinium macrocarpon (cranberry) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
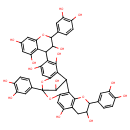
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Epicatechin-(4b->6)-epicatechin-(2b->7,4b->8)-epicatechin | Generator | | Epicatechin-(4β->6)-epicatechin-(2β->7,4β->8)-epicatechin | Generator |
|
|---|
| Chemical Formula | C45H36O18 |
|---|
| Average Molecular Mass | 864.757 g/mol |
|---|
| Monoisotopic Mass | 864.190 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5,13-bis(3,4-dihydroxyphenyl)-18-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]-4,12,14-trioxapentacyclo[11.7.1.0²,¹¹.0³,⁸.0¹⁵,²⁰]henicosa-2(11),3(8),9,15(20),16,18-hexaene-6,9,17,19,21-pentol |
|---|
| Traditional Name | 5,13-bis(3,4-dihydroxyphenyl)-18-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]-4,12,14-trioxapentacyclo[11.7.1.0²,¹¹.0³,⁸.0¹⁵,²⁰]henicosa-2(11),3(8),9,15(20),16,18-hexaene-6,9,17,19,21-pentol |
|---|
| SMILES | OC1CC2=C(OC1C1=CC(O)=C(O)C=C1)C1=C(OC3(OC4=C(C1C3O)C(O)=C(C1C(O)C(OC3=CC(O)=CC(O)=C13)C1=CC(O)=C(O)C=C1)C(O)=C4)C1=CC(O)=C(O)C=C1)C=C2O |
|---|
| InChI Identifier | InChI=1S/C45H36O18/c46-18-10-27(54)33-30(11-18)60-42(16-2-5-21(48)25(52)8-16)40(58)37(33)34-28(55)14-31-35(39(34)57)38-36-32(63-45(62-31,44(38)59)17-3-6-22(49)26(53)9-17)13-23(50)19-12-29(56)41(61-43(19)36)15-1-4-20(47)24(51)7-15/h1-11,13-14,29,37-38,40-42,44,46-59H,12H2 |
|---|
| InChI Key | SSOWHRNCDFFKAK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biflavonoids and polyflavonoids. These are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Biflavonoids and polyflavonoids |
|---|
| Direct Parent | Biflavonoids and polyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - B-type proanthocyanidin
- A-type proanthocyanidin
- Bi- and polyflavonoid skeleton
- Proanthocyanidin
- Catechin
- Pyranoflavonoid
- 3'-hydroxyflavonoid
- Flavan-3-ol
- 3-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Hydroxyflavonoid
- Flavan
- Pyranochromene
- 1-benzopyran
- Benzopyran
- Chromane
- Catechol
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Ketal
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Ether
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0292-0211003490-f40e2800ee162693329e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06r5-0600084940-c2014724ebb530c36f76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0910020110-8159217f4a14a06333d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0111000290-14f0e1f3d1b46e11e789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w2j-0920140450-d45ea33b8b96cf6db20b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002r-0960100000-8c69407a1062205d124a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000090-337930111bacd434d3e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000000590-5ceefab6afee18325d0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ti-0120000940-8209959318b1d5a284ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000000390-07cb5f550a83e1aa110b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0000070690-f679cb9cbed6d4951a51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05i9-0900010560-f46a9f3c7be0a2afe1c4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035504 |
|---|
| FooDB ID | FDB014194 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013941 |
|---|
| ChEBI ID | 169322 |
|---|
| PubChem Compound ID | 14015928 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|