| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:06:26 UTC |
|---|
| Update Date | 2016-11-09 01:18:52 UTC |
|---|
| Accession Number | CHEM029340 |
|---|
| Identification |
|---|
| Common Name | Promucosine |
|---|
| Class | Small Molecule |
|---|
| Description | Promucosine is found in beverages. Promucosine is an alkaloid from Annona purpurea (soncoya |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
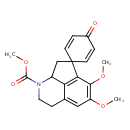
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 10',11'-dimethoxy-4-oxo-5'-azaspiro[cyclohexane-1,2'-tricyclo[6.3.1.0⁴,¹²]dodecane]-1'(12'),2,5,8',10'-pentaene-5'-carboxylic acid | HMDB |
|
|---|
| Chemical Formula | C20H21NO5 |
|---|
| Average Molecular Mass | 355.384 g/mol |
|---|
| Monoisotopic Mass | 355.142 g/mol |
|---|
| CAS Registry Number | 275355-87-8 |
|---|
| IUPAC Name | methyl 10',11'-dimethoxy-4-oxo-5'-azaspiro[cyclohexane-1,2'-tricyclo[6.3.1.0⁴,¹²]dodecane]-1'(12'),2,5,8',10'-pentaene-5'-carboxylate |
|---|
| Traditional Name | methyl 10',11'-dimethoxy-4-oxo-5'-azaspiro[cyclohexane-1,2'-tricyclo[6.3.1.0⁴,¹²]dodecane]-1'(12'),2,5,8',10'-pentaene-5'-carboxylate |
|---|
| SMILES | COC(=O)N1CCC2=CC(OC)=C(OC)C3=C2C1CC31C=CC(=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C20H21NO5/c1-24-15-10-12-6-9-21(19(23)26-3)14-11-20(7-4-13(22)5-8-20)17(16(12)14)18(15)25-2/h4-5,7-8,10,14H,6,9,11H2,1-3H3 |
|---|
| InChI Key | RRWYPLCSLKEVAO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proaporphines. These are benzylisoquinoline derivatives characterized by the presence of a spirocyclohexane ring that can occur in various oxidation levels, from cyclohexadienone to cyclohexanol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Proaporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Proaporphines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proaporphine
- Tetrahydroisoquinoline
- Indane
- Anisole
- Alkyl aryl ether
- Benzenoid
- Methylcarbamate
- Carbamic acid ester
- Cyclic ketone
- Carbonic acid derivative
- Ketone
- Ether
- Organoheterocyclic compound
- Azacycle
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01yy-0069000000-110fa642fd57962b902a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-6c2decad0f7309798ee5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0079000000-d513b278e27a820dc280 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-032c-2090000000-c8148f11189e0a71f253 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uk9-0009000000-cd2fc9b42733401fce6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0019000000-2c12f5e0a12c414fead2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2097000000-39067db671ae6ba03ee9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-3f012a8ceb78f6daa2f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-0090000000-a7e5f45280c3cc9f1a35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uec-1092000000-773b5d6d1edb73352973 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-d71201421eba67513e90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ac0-0049000000-6ccfb231ff26ffacf6aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ff1-1093000000-6eb98ec2efe1cf2f5ce8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035438 |
|---|
| FooDB ID | FDB014119 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027161 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10349245 |
|---|
| ChEBI ID | 174769 |
|---|
| PubChem Compound ID | 131751753 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|