| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:05:26 UTC |
|---|
| Update Date | 2016-11-09 01:18:51 UTC |
|---|
| Accession Number | CHEM029321 |
|---|
| Identification |
|---|
| Common Name | Pyranodelphinin B |
|---|
| Class | Small Molecule |
|---|
| Description | Found in polluted water, causes objectionable odour of water supplies. Implicated in off-flavour of freshwater fish and shellfish |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
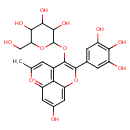
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H23O12 |
|---|
| Average Molecular Mass | 503.432 g/mol |
|---|
| Monoisotopic Mass | 503.119 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 11-hydroxy-3-methyl-6-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-7-(3,4,5-trihydroxyphenyl)-2λ⁴,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1,3,5(13),6,9,11-hexaen-2-ylium |
|---|
| Traditional Name | 11-hydroxy-3-methyl-6-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-7-(3,4,5-trihydroxyphenyl)-2λ⁴,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1,3,5(13),6,9,11-hexaen-2-ylium |
|---|
| SMILES | CC1=CC2=C3C(OC(C4=CC(O)=C(O)C(O)=C4)=C2OC2OC(CO)C(O)C(O)C2O)=CC(O)=CC3=[O+]1 |
|---|
| InChI Identifier | InChI=1S/C24H22O12/c1-8-2-11-17-14(33-8)5-10(26)6-15(17)34-22(9-3-12(27)18(29)13(28)4-9)23(11)36-24-21(32)20(31)19(30)16(7-25)35-24/h2-6,16,19-21,24-25,30-32H,7H2,1H3,(H3-,26,27,28,29)/p+1 |
|---|
| InChI Key | GDTLCJKHXQSPKO-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Bicyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bicyclic monoterpenoid
- Bornane monoterpenoid
- Tertiary alcohol
- Cyclic alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05g0-9201700000-a78cbbf9eb0bee999a05 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-053r-4610119000-dbca95c6aaeb968739f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0100090000-6fc16d369a93ee6fb450 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2500390000-da0589b846941d4d54ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-7902000000-1fb621a638a074ba0ea6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2300090000-44659335dbb0ceaaccfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-7900080000-c915cdbd834a93ab9044 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9200000000-f24b700d63c5558fc4fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009120000-928838aa0ebc2d648241 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0009000000-4bd256660b9de76724c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-3149100000-c8f063d9a9da3ac91b76 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035417 |
|---|
| FooDB ID | FDB014095 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 16024 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 16913 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|