| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:03:33 UTC |
|---|
| Update Date | 2016-11-09 01:18:51 UTC |
|---|
| Accession Number | CHEM029276 |
|---|
| Identification |
|---|
| Common Name | Cytochalasin Npho |
|---|
| Class | Small Molecule |
|---|
| Description | Cytochalasin Npho is a mycotoxin from Phomopsis sp |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
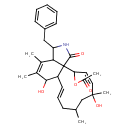
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cytochalasin n? | HMDB | | 3-Benzyl-1,6,12-trihydroxy-4,5,10,12-tetramethyl-3H,6H,6ah,9H,10H,11H,12H,15H,15BH-cycloundeca[e]isoindol-15-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C30H39NO5 |
|---|
| Average Molecular Mass | 493.634 g/mol |
|---|
| Monoisotopic Mass | 493.283 g/mol |
|---|
| CAS Registry Number | 108050-28-8 |
|---|
| IUPAC Name | 3-benzyl-6,12-dihydroxy-4,5,10,12-tetramethyl-1-oxo-1H,2H,3H,6H,6aH,9H,10H,11H,12H,15H,15bH-cycloundeca[e]isoindol-15-yl acetate |
|---|
| Traditional Name | 3-benzyl-6,12-dihydroxy-4,5,10,12-tetramethyl-1-oxo-2H,3H,6H,6aH,9H,10H,11H,15H,15bH-cycloundeca[e]isoindol-15-yl acetate |
|---|
| SMILES | CC1C\C=C\C2C(O)C(C)=C(C)C3C(CC4=CC=CC=C4)NC(=O)C23C(OC(C)=O)\C=C/C(C)(O)C1 |
|---|
| InChI Identifier | InChI=1S/C30H39NO5/c1-18-10-9-13-23-27(33)20(3)19(2)26-24(16-22-11-7-6-8-12-22)31-28(34)30(23,26)25(36-21(4)32)14-15-29(5,35)17-18/h6-9,11-15,18,23-27,33,35H,10,16-17H2,1-5H3,(H,31,34)/b13-9+,15-14- |
|---|
| InChI Key | WFSYATBEJTUDQA-WISUYLHISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cytochalasans. These are fungal metabolites structurally characterized by the presence of an isoindolone nucleus fused to a macrocyclic ring, which can either a lactone, as in cytochalasin B, a carbonate, as in cytochalasin E, or a carbocycle, as in cytochalasin D, H, and K. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Cytochalasans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Cytochalasans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carbocyclic cytochalasan skeleton
- Cytochalasan
- Isoindolone
- Isoindoline
- Isoindole
- Isoindole or derivatives
- Monocyclic benzene moiety
- Benzenoid
- Pyrrolidone
- 2-pyrrolidone
- Pyrrolidine
- Tertiary alcohol
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid ester
- Lactam
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0036-9001700000-ccb3b677aaf655efdd52 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fr-8000093000-2672d3ce2062c3418e0b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0000900000-a887a58c18dbefb347da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003r-1000900000-6a1c878ec99dc2b45557 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pbc-8902400000-8e4f29fec6fd370499bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1000900000-1191b7343df6d6b44872 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0khc-2000900000-32310d51739119b187f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9036300000-5183c0a016d1038c2f19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-c01bbbf5bed889264ddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-c01bbbf5bed889264ddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-2dee1995aa51e221300f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00mo-0000900000-b7d2c978208cf4a488fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0000900000-ccf8da00f7c7629e69a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-2206900000-a6b42b2c43ecc50977d3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035367 |
|---|
| FooDB ID | FDB014040 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00011339 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013911 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751725 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|