| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:02:15 UTC |
|---|
| Update Date | 2016-11-09 01:18:50 UTC |
|---|
| Accession Number | CHEM029244 |
|---|
| Identification |
|---|
| Common Name | Ganodermic acid Jb |
|---|
| Class | Small Molecule |
|---|
| Description | Metabolite of Ganoderma lucidum (reishi). Ganodermic acid Ja is found in mushrooms. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
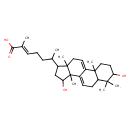
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ganodermate JB | Generator | | (2E)-6-{5,12-dihydroxy-2,6,6,11,15-pentamethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(17),9-dien-14-yl}-2-methylhept-2-enoate | Generator |
|
|---|
| Chemical Formula | C30H46O4 |
|---|
| Average Molecular Mass | 470.684 g/mol |
|---|
| Monoisotopic Mass | 470.340 g/mol |
|---|
| CAS Registry Number | 112430-68-9 |
|---|
| IUPAC Name | (2E)-6-{5,12-dihydroxy-2,6,6,11,15-pentamethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(17),9-dien-14-yl}-2-methylhept-2-enoic acid |
|---|
| Traditional Name | (2E)-6-{5,12-dihydroxy-2,6,6,11,15-pentamethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(17),9-dien-14-yl}-2-methylhept-2-enoic acid |
|---|
| SMILES | CC(CC\C=C(/C)C(O)=O)C1CC(O)C2(C)C3=CCC4C(C)(C)C(O)CCC4(C)C3=CCC12C |
|---|
| InChI Identifier | InChI=1S/C30H46O4/c1-18(9-8-10-19(2)26(33)34)22-17-25(32)30(7)21-11-12-23-27(3,4)24(31)14-15-28(23,5)20(21)13-16-29(22,30)6/h10-11,13,18,22-25,31-32H,8-9,12,14-17H2,1-7H3,(H,33,34)/b19-10+ |
|---|
| InChI Key | VFCVARJMDQZNKD-VXLYETTFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kdl-0012900000-7d53cef5b8951720cc4f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-1010059000-a7efd85f2ea53cb78276 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0001900000-307c59222e43c04f3b48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pdr-0005900000-7e02b0e59a49b20f21f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4519800000-117ee14bce4bd0024d49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-b8791dd205737681b5d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0lfr-0000900000-47a388826c2f989d684a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4002900000-be583da8afa84a50313c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01vk-9208600000-29cfb652771c6b4a11a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-9005200000-7c844a0245f8ae2fa2d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9213000000-68c9e013c9870b09e8c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-0000900000-a01d51e0633a69dfc0ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-0003900000-2ac968dbe5e06e27f77a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-8009800000-2dadf97c416985bfec13 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035334 |
|---|
| FooDB ID | FDB014006 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14325145 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|