| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:01:16 UTC |
|---|
| Update Date | 2016-11-09 01:18:50 UTC |
|---|
| Accession Number | CHEM029222 |
|---|
| Identification |
|---|
| Common Name | Galanal A |
|---|
| Class | Small Molecule |
|---|
| Description | Galanal B is found in herbs and spices. Galanal B is a constituent of seeds of Alpinia galanga (greater galangal). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
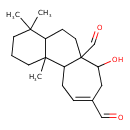
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Mass | 318.450 g/mol |
|---|
| Monoisotopic Mass | 318.219 g/mol |
|---|
| CAS Registry Number | 104086-74-0 |
|---|
| IUPAC Name | 7-hydroxy-4,4,11b-trimethyl-1H,2H,3H,4H,4aH,5H,6H,6aH,7H,8H,11H,11aH,11bH-cyclohepta[a]naphthalene-6a,9-dicarbaldehyde |
|---|
| Traditional Name | 7-hydroxy-4,4,11b-trimethyl-1H,2H,3H,4aH,5H,6H,7H,8H,11H,11aH-cyclohepta[a]naphthalene-6a,9-dicarbaldehyde |
|---|
| SMILES | CC1(C)CCCC2(C)C1CCC1(C=O)C2CC=C(CC1O)C=O |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-18(2)8-4-9-19(3)15(18)7-10-20(13-22)16(19)6-5-14(12-21)11-17(20)23/h5,12-13,15-17,23H,4,6-11H2,1-3H3 |
|---|
| InChI Key | UDKRLAJJSYRYRU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as secondary alcohols. Secondary alcohols are compounds containing a secondary alcohol functional group, with the general structure HOC(R)(R') (R,R'=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Secondary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aldehyde
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-1292000000-fb90e1b1161e73bbd5f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0200-2219000000-5aac223f0dc725bdf95d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0019000000-3af6fbaf1479a05f1582 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-4369000000-6e919ad1a64d0283fc83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05uv-7590000000-e92bf32cdb979438bf87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-d8f40a2f1a823635fe92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0089000000-fb411e22efe49c8fdd17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abl-1091000000-1b9bc45f4738202c4b2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0019000000-f78677a0721273510002 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-0089000000-c2a5f86b39f6d906dc42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02ti-4491000000-b5fefc987f2c054aac07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-840c705ba16b2416bc9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0392000000-9701e167752ced2c94f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mw-4910000000-d3f7e0e575706a198c2b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035311 |
|---|
| FooDB ID | FDB013980 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034847 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013900 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14330184 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|