| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:01:04 UTC |
|---|
| Update Date | 2016-11-09 01:18:50 UTC |
|---|
| Accession Number | CHEM029217 |
|---|
| Identification |
|---|
| Common Name | Ganoderic acid C2 |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of paprika (Capsicum annuum). Cryptocapsin is found in many foods, some of which are pepper (c. annuum), herbs and spices, red bell pepper, and orange bell pepper. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
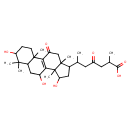
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3's,5'r)-3'-Hydroxy-beta,kappa-caroten-6'-one | HMDB | | 3'-Hydroxy-b,K-caroten-6'-one | HMDB | | Kryptocapsin | HMDB | | Lanost-8-en-26-Oic acid, 3,7,15-trihydroxy-11,23-dioxo-, (3beta,15alpha)- (9ci) | HMDB | | 2-Methyl-4-oxo-6-{5,9,12-trihydroxy-2,6,6,11,15-pentamethyl-17-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-1(10)-en-14-yl}heptanoate | Generator | | Ganoderic acid C2 | MeSH | | Ganoderate C2 | Generator |
|
|---|
| Chemical Formula | C30H46O7 |
|---|
| Average Molecular Mass | 518.682 g/mol |
|---|
| Monoisotopic Mass | 518.324 g/mol |
|---|
| CAS Registry Number | 98296-48-1 |
|---|
| IUPAC Name | 2-methyl-4-oxo-6-{5,9,12-trihydroxy-2,6,6,11,15-pentamethyl-17-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-1(10)-en-14-yl}heptanoic acid |
|---|
| Traditional Name | 2-methyl-4-oxo-6-{5,9,12-trihydroxy-2,6,6,11,15-pentamethyl-17-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-1(10)-en-14-yl}heptanoic acid |
|---|
| SMILES | CC(CC(=O)CC(C)C(O)=O)C1CC(O)C2(C)C3=C(C(=O)CC12C)C1(C)CCC(O)C(C)(C)C1CC3O |
|---|
| InChI Identifier | InChI=1S/C30H46O7/c1-15(10-17(31)11-16(2)26(36)37)18-12-23(35)30(7)25-19(32)13-21-27(3,4)22(34)8-9-28(21,5)24(25)20(33)14-29(18,30)6/h15-16,18-19,21-23,32,34-35H,8-14H2,1-7H3,(H,36,37) |
|---|
| InChI Key | RERVSJVGWKIGTJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Cyclopentanol
- Alpha,beta-unsaturated ketone
- Enone
- Cyclic alcohol
- Acryloyl-group
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fe0-1115930000-a60eb9896660bfb73644 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0002-4210149000-b7020da91a0e99db76a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-1001970000-41ed07f24bfa88e519af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0560-1001910000-2164bec2f55b527238c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pbl-5103900000-6853f406cf76b7775489 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0000980000-4eaad17d545592fe3eed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fs-5100920000-1b3588e4387ca4a263e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adr-9111710000-f8a579490d4cf6afa293 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000190000-4cceff31bced10c3ec5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-2006910000-e2230e350e1f27cc806e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ri-6009700000-e37e86325c5a5a78c84f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00sc-0209220000-1e14c720ff2737ab0802 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01x9-5609210000-61552fd53ad5adfde209 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01vx-8409100000-5c30579545f14a906d3b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036923 |
|---|
| FooDB ID | FDB015892 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00022984 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752094 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|