| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:57:50 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029147 |
|---|
| Identification |
|---|
| Common Name | Isocyclocalamin |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of a Citrus reticulata/Fortunella hybrid. Isocyclocalamin is found in citrus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
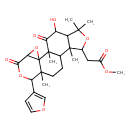
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 2-[7-(furan-3-yl)-17-hydroxy-1,8,12,15,15-pentamethyl-5,18-dioxo-3,6,14-trioxapentacyclo[9.7.0.0²,⁴.0²,⁸.0¹²,¹⁶]octadecan-13-yl]acetic acid | Generator |
|
|---|
| Chemical Formula | C27H34O9 |
|---|
| Average Molecular Mass | 502.554 g/mol |
|---|
| Monoisotopic Mass | 502.220 g/mol |
|---|
| CAS Registry Number | 111004-32-1 |
|---|
| IUPAC Name | methyl 2-[7-(furan-3-yl)-17-hydroxy-1,8,12,15,15-pentamethyl-5,18-dioxo-3,6,14-trioxapentacyclo[9.7.0.0²,⁴.0²,⁸.0¹²,¹⁶]octadecan-13-yl]acetate |
|---|
| Traditional Name | methyl 2-[7-(furan-3-yl)-17-hydroxy-1,8,12,15,15-pentamethyl-5,18-dioxo-3,6,14-trioxapentacyclo[9.7.0.0²,⁴.0²,⁸.0¹²,¹⁶]octadecan-13-yl]acetate |
|---|
| SMILES | COC(=O)CC1OC(C)(C)C2C(O)C(=O)C3(C)C(CCC4(C)C(OC(=O)C5OC345)C3=COC=C3)C12C |
|---|
| InChI Identifier | InChI=1S/C27H34O9/c1-23(2)18-17(29)19(30)26(5)14(25(18,4)15(35-23)11-16(28)32-6)7-9-24(3)20(13-8-10-33-12-13)34-22(31)21-27(24,26)36-21/h8,10,12,14-15,17-18,20-21,29H,7,9,11H2,1-6H3 |
|---|
| InChI Key | BPAWUWIFAKISJD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid lactones. These are sterol lipids containing a lactone moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid lactones |
|---|
| Direct Parent | Steroid lactones |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-5052900000-1fe7169fc861db19cf02 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-074i-7154890000-4e26a5ddd7f05ea070df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uki-0000930000-bc6601447e730b6ff2c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gba-0002900000-c05779f48a7afc06d40f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0532-9314100000-1ae2af623d5f1f5b3771 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-1000940000-530ddafec2d7259be3e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0le9-2000910000-25cdbb8e7ecc87c41b08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05be-9001700000-14e4f9ec98d70740fe47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000490000-9acb036d42029e5a6454 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6y-0004920000-876d18b435e582cfd33e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-2003900000-3a9374c14553d8a63aee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000290000-0ee819f2d9f015132136 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-0000920000-eb006d27b30129972442 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k92-3314910000-34ebf001ae002c815270 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035222 |
|---|
| FooDB ID | FDB013871 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13857942 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|