| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:57:41 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029143 |
|---|
| Identification |
|---|
| Common Name | Cinereain |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from Botrytis cinerea on sunflower seed. Cinereain is found in cereals and cereal products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
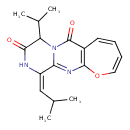
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10,11-Dihydro-8-(1-methylethyl)-11-(2-methylpropylidene)-6H-oxepino[2,3-D]pyrazino[1,2-a]pyrimidine-6,9(8H)-dione, 9ci | HMDB |
|
|---|
| Chemical Formula | C18H21N3O3 |
|---|
| Average Molecular Mass | 327.378 g/mol |
|---|
| Monoisotopic Mass | 327.158 g/mol |
|---|
| CAS Registry Number | 117013-51-1 |
|---|
| IUPAC Name | (4E)-4-(2-methylpropylidene)-7-(propan-2-yl)-15-oxa-2,5,8-triazatricyclo[8.5.0.0³,⁸]pentadeca-1(10),2,11,13-tetraene-6,9-dione |
|---|
| Traditional Name | (4E)-7-isopropyl-4-(2-methylpropylidene)-15-oxa-2,5,8-triazatricyclo[8.5.0.0³,⁸]pentadeca-1(10),2,11,13-tetraene-6,9-dione |
|---|
| SMILES | CC(C)\C=C1\NC(=O)C(C(C)C)N2C(=O)C3=C(OC=CC=C3)N=C12 |
|---|
| InChI Identifier | InChI=1S/C18H21N3O3/c1-10(2)9-13-15-20-17-12(7-5-6-8-24-17)18(23)21(15)14(11(3)4)16(22)19-13/h5-11,14H,1-4H3,(H,19,22)/b13-9+ |
|---|
| InChI Key | CCHUDPANZXHQCS-UKTHLTGXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Vinylogous ester
- Heteroaromatic compound
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- Oxacycle
- Azacycle
- Carboxylic acid derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-075d-2294000000-9e3ecd38bf87ae1652dd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0019000000-79c9743afc472c38e8bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3139000000-6174911fe460746b7737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0nmr-9650000000-73fbffdf511d6bec0ab9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0029000000-51fb249be555aca7591d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-7493000000-0209f631ef4f9bf3111d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-3970000000-498bdea4f7c821680bdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0039000000-133fca5f99ad6bf17d7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0094000000-f467d8886ec75d541bf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-3191000000-226d57eaa764ca775599 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-5b65f30965dc13ccd7b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0019000000-5837a0dd8e829df0106b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-2397000000-e87a4b491313e3d7c1b1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035218 |
|---|
| FooDB ID | FDB013866 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 51340308 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|