| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:56:52 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029123 |
|---|
| Identification |
|---|
| Common Name | 1-(9H-Pyrido[3,4-b]indol-1-yl)-1,4-butanediol |
|---|
| Class | Small Molecule |
|---|
| Description | 1-(9H-Pyrido[3,4-b]indol-1-yl)-1,4-butanediol is maillard produced from xylose and tryptophan. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
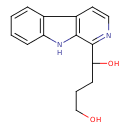
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(1,4-Dihydroxybutyl)-9H-pyrido[3,4-b]indole | HMDB | | 1-(9H-pyrido[3,4-b]indol-1-yl)-1,4-Butanediol, 9ci | HMDB |
|
|---|
| Chemical Formula | C15H16N2O2 |
|---|
| Average Molecular Mass | 256.300 g/mol |
|---|
| Monoisotopic Mass | 256.121 g/mol |
|---|
| CAS Registry Number | 220345-68-6 |
|---|
| IUPAC Name | 1-{9H-pyrido[3,4-b]indol-1-yl}butane-1,4-diol |
|---|
| Traditional Name | 1-{9H-pyrido[3,4-b]indol-1-yl}butane-1,4-diol |
|---|
| SMILES | OCCCC(O)C1=NC=CC2=C1NC1=C2C=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C15H16N2O2/c18-9-3-6-13(19)15-14-11(7-8-16-15)10-4-1-2-5-12(10)17-14/h1-2,4-5,7-8,13,17-19H,3,6,9H2 |
|---|
| InChI Key | SPYBYBYFMPTBMD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harman
- Beta-carboline
- Pyridoindole
- Indole
- Indole or derivatives
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Secondary alcohol
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0554-9640000000-a9405d45e4cde390855a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-4193000000-8c91bb64240a89a0f6b3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-87d10c2f34e27d176077 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-1090000000-b27c271055682f0626c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9750000000-48d9bf3e2d291e3832ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-8058b8e1af49c2022119 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ap0-1390000000-b3815abf5e403854d480 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-5920000000-1eea6ef1287c115475cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-3c93e7d59fd6f4fb59b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-0590000000-69c18f7b407475ed35e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-39a3a73facb9b920ee38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-a9bdbb8ed185931a42da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0290000000-680a6e90f9be6175a66d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-017i-0920000000-c9ab0709ad3c29798695 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035193 |
|---|
| FooDB ID | FDB013839 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013869 |
|---|
| ChEBI ID | 172497 |
|---|
| PubChem Compound ID | 15394712 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|