| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:56:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029120 |
|---|
| Identification |
|---|
| Common Name | Artonin F |
|---|
| Class | Small Molecule |
|---|
| Description | Artonin F is found in breadfruit. Artonin F is a constituent of Artocarpus communis (breadfruit). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
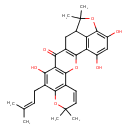
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C30H30O7 |
|---|
| Average Molecular Mass | 502.555 g/mol |
|---|
| Monoisotopic Mass | 502.199 g/mol |
|---|
| CAS Registry Number | 129683-94-9 |
|---|
| IUPAC Name | 12,21,23-trihydroxy-8,8,18,18-tetramethyl-11-(3-methylbut-2-en-1-yl)-3,9,19-trioxahexacyclo[15.6.1.0²,¹⁵.0⁴,¹³.0⁵,¹⁰.0²⁰,²⁴]tetracosa-1(24),2(15),4(13),5(10),6,11,20,22-octaen-14-one |
|---|
| Traditional Name | artonin F |
|---|
| SMILES | CC(C)=CCC1=C(O)C2=C(OC3=C(CC4C5=C3C(O)=CC(O)=C5OC4(C)C)C2=O)C2=C1OC(C)(C)C=C2 |
|---|
| InChI Identifier | InChI=1S/C30H30O7/c1-13(2)7-8-14-23(33)22-24(34)16-11-17-20-21(18(31)12-19(32)28(20)37-30(17,5)6)27(16)35-26(22)15-9-10-29(3,4)36-25(14)15/h7,9-10,12,17,31-33H,8,11H2,1-6H3 |
|---|
| InChI Key | GKWNQOINHKVKOT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoxanthones. These are organic aromatic compounds containing a pyran or a hydrogenated derivative fused to a xanthone ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Pyranoxanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranoxanthone
- Naphthopyranone
- Naphthopyran
- Pyranochromene
- 2,2-dimethyl-1-benzopyran
- Chromone
- 1-naphthol
- Naphthalene
- Coumaran
- Alkyl aryl ether
- Pyranone
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Pyran
- Vinylogous acid
- Heteroaromatic compound
- Polyol
- Oxacycle
- Ether
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-2010900000-894b679da4ecccb6ab84 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-2103029000-74e85e677504c2962917 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1000890000-91be348d980d829445bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0mi2-2000910000-580b6bc418795d1276f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-5012900000-edc6d990665b7adb1473 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000190000-7f355ecf8fba9337bbca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0010980000-3912a2c36f2adeaa2a60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-067r-0142900000-7d345ce4231a982b9ed4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000090000-27a32bd6c97a965edc44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000090000-27a32bd6c97a965edc44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-0090010000-7e9d5fac0bdd16aa0613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000090000-db49500cd3a1fa636786 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000090000-db49500cd3a1fa636786 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-0090250000-51ff50700d8384e3ac9c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035187 |
|---|
| FooDB ID | FDB013833 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00013489 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10211392 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14680593 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|