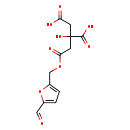| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:56:18 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029112 |
|---|
| Identification |
|---|
| Common Name | Mumefural |
|---|
| Class | Small Molecule |
|---|
| Description | Mumefural is isolated from fruit juice concentrate of Prunus mume (Japanese apricot |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-1,2,3-propanetricarboxylic acid, 1-[(5-formyl-2-furanyl)methyl] ester, 9ci | HMDB | | 2-{2-[(5-formylfuran-2-yl)methoxy]-2-oxoethyl}-2-hydroxybutanedioate | Generator | | Mumefural | MeSH |
|
|---|
| Chemical Formula | C12H12O9 |
|---|
| Average Molecular Mass | 300.218 g/mol |
|---|
| Monoisotopic Mass | 300.048 g/mol |
|---|
| CAS Registry Number | 222973-44-6 |
|---|
| IUPAC Name | 2-{2-[(5-formylfuran-2-yl)methoxy]-2-oxoethyl}-2-hydroxybutanedioic acid |
|---|
| Traditional Name | 2-{2-[(5-formylfuran-2-yl)methoxy]-2-oxoethyl}-2-hydroxybutanedioic acid |
|---|
| SMILES | OC(=O)CC(O)(CC(=O)OCC1=CC=C(O1)C=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C12H12O9/c13-5-7-1-2-8(21-7)6-20-10(16)4-12(19,11(17)18)3-9(14)15/h1-2,5,19H,3-4,6H2,(H,14,15)(H,17,18) |
|---|
| InChI Key | FYDIRKLRXHXXHY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tricarboxylic acids and derivatives |
|---|
| Direct Parent | Tricarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tricarboxylic acid or derivatives
- Fatty acid ester
- Aryl-aldehyde
- Alpha-hydroxy acid
- Hydroxy acid
- Fatty acyl
- Furan
- Heteroaromatic compound
- Tertiary alcohol
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Aldehyde
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-3910000000-44760b535b08161b7eb2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a4i-3923210000-74c51ff93a501bc09d7a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kur-0982000000-4a3f14bed504c0c440bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-2920000000-d89b90e257b732fd742b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0561-7910000000-5fa6ddef08401c3c6d63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0541-2950000000-249929a32993a4e345f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-2920000000-552aece82ed062fb0463 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-8900000000-b6f4ad45d4e9c8443175 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0910000000-ca02eab858d029ddb891 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-2910000000-825852523599408bcf02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-9000000000-4bd747cd005d33b542d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0api-0591000000-bfd834cadb7821cfcb1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054t-7920000000-187dd359a6eb68a85fa6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9200000000-604e6183d6abb34b8985 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035179 |
|---|
| FooDB ID | FDB013819 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057260 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8955855 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10780542 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|