| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:56:12 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029109 |
|---|
| Identification |
|---|
| Common Name | 2-Amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine is found in animal foods. 2-Amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine is isolated from cooked meat |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
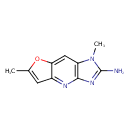
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,6-Dimethyl-1H-furo[2,3-b]imidazo[4,5-e]pyridin-2-amine, 9ci | HMDB | | 2-Amino-(1,6-dimethylfuro(3,2-e)imidazo(4,5-b))pyridine | MeSH | | 2-Amino-dfip | MeSH |
|
|---|
| Chemical Formula | C10H10N4O |
|---|
| Average Molecular Mass | 202.213 g/mol |
|---|
| Monoisotopic Mass | 202.085 g/mol |
|---|
| CAS Registry Number | 132898-08-9 |
|---|
| IUPAC Name | 6,11-dimethyl-10-oxa-2,4,6-triazatricyclo[7.3.0.0³,⁷]dodeca-1,3(7),4,8,11-pentaen-5-amine |
|---|
| Traditional Name | 6,11-dimethyl-10-oxa-2,4,6-triazatricyclo[7.3.0.0³,⁷]dodeca-1,3(7),4,8,11-pentaen-5-amine |
|---|
| SMILES | CN1C(N)=NC2=C1C=C1OC(C)=CC1=N2 |
|---|
| InChI Identifier | InChI=1S/C10H10N4O/c1-5-3-6-8(15-5)4-7-9(12-6)13-10(11)14(7)2/h3-4H,1-2H3,(H2,11,12,13) |
|---|
| InChI Key | MUFPYNUSILUNGN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazopyridines. These are organic polycyclic compounds containing an imidazole ring fused to a pyridine ring. Imidazole is 5-membered ring consisting of three carbon atoms, and two nitrogen centers at the 1- and 3-positions. Pyridine is a 6-membered ring consisting of five carbon atoms and one nitrogen center. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyridines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Imidazopyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furopyridine
- Imidazopyridine
- Aminoimidazole
- Pyridine
- N-substituted imidazole
- Azole
- Furan
- Imidazole
- Heteroaromatic compound
- Oxacycle
- Azacycle
- Amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0w2a-0920000000-1130384343b4dea7a3f1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-ca41536b94b6eb75559f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0090000000-7bc4a0bc50899d912fda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01qi-1900000000-af26e31708b5419c8c92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-6778da7c2f335253c69e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0290000000-806320ddc8ce06835bbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-4900000000-0365a85116e4b5df06b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-73a2df502e09c0542e48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0090000000-f9446ff851dcbadbb12b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fki-0920000000-ff89fb2aad4f7a9c5e61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-e7482f51c32ab39ecfb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0290000000-790f10d7d9baea18b6f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dj-0910000000-33fb6404ab36bb195e34 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035175 |
|---|
| FooDB ID | FDB013815 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 29787112 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 71313206 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|