| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:56:10 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029108 |
|---|
| Identification |
|---|
| Common Name | [3-[1-Formyl-2-(2-furanyl)ethenyl]]-2-(2-furanyl)-5-(2-furanylmethylene)-4,5-dihydro-a-methyl-4-oxo-1H-pyrrole-1-acetic acid, 9CI |
|---|
| Class | Small Molecule |
|---|
| Description | [3-[1-Formyl-2-(2-furanyl)ethenyl]]-2-(2-furanyl)-5-(2-furanylmethylene)-4,5-dihydro-a-methyl-4-oxo-1H-pyrrole-1-acetic acid, 9CI is a maillard product from reaction of L-alanine and 2-Furancarboxaldehyde FTR29-Z |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
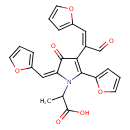
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [3-[1-Formyl-2-(2-furanyl)ethenyl]]-2-(2-furanyl)-5-(2-furanylmethylene)-4,5-dihydro-a-methyl-4-oxo-1H-pyrrole-1-acetate, 9ci | Generator | | 4-Hydroxy-N-(2-diethylaminoethyl)benzamide | HMDB | | N-(2-(diethylamino)Ethyl)-4-hydroxybenzamide hydrochloride | HMDB | | P-Hydroxy-N-(2-diethylaminoethyl)benzamide hydrochloride | HMDB | | 2-[(2E)-5-(Furan-2-yl)-4-[(1E)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl]-2-[(furan-2-yl)methylidene]-3-oxo-2,3-dihydro-1H-pyrrol-1-yl]propanoate | Generator |
|
|---|
| Chemical Formula | C23H17NO7 |
|---|
| Average Molecular Mass | 419.384 g/mol |
|---|
| Monoisotopic Mass | 419.101 g/mol |
|---|
| CAS Registry Number | 198414-08-3 |
|---|
| IUPAC Name | 2-[(2E)-5-(furan-2-yl)-4-[(1E)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl]-2-(furan-2-ylmethylidene)-3-oxo-2,3-dihydro-1H-pyrrol-1-yl]propanoic acid |
|---|
| Traditional Name | 2-[(5E)-2-(furan-2-yl)-3-[(1E)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl]-5-(furan-2-ylmethylidene)-4-oxopyrrol-1-yl]propanoic acid |
|---|
| SMILES | CC(N1\C(=C\C2=CC=CO2)C(=O)C(\C(C=O)=C/C2=CC=CO2)=C1C1=CC=CO1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H17NO7/c1-14(23(27)28)24-18(12-17-6-3-9-30-17)22(26)20(21(24)19-7-4-10-31-19)15(13-25)11-16-5-2-8-29-16/h2-14H,1H3,(H,27,28)/b15-11-,18-12+ |
|---|
| InChI Key | WKLHCENPFNYZSQ-DARJFKRNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alanine and derivatives. Alanine and derivatives are compounds containing alanine or a derivative thereof resulting from reaction of alanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alanine or derivatives
- Alpha-amino acid
- Alpha,beta-unsaturated aldehyde
- Enal
- Furan
- Pyrroline
- Vinylogous amide
- Heteroaromatic compound
- Amino acid
- Ketone
- Tertiary amine
- Tertiary aliphatic amine
- Cyclic ketone
- Oxacycle
- Carboxylic acid
- Enamine
- Azacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organopnictogen compound
- Aldehyde
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Amine
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01vo-4149100000-bb0d8893300f4a312258 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00fr-9327500000-d6df714ac43a931a4889 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009800000-1f54973bdf1ea604d5d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2129100000-cd1cf8d7efbc24e98b6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2129000000-da317b6ccee2673e4f98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0002900000-619a806b7833bca918bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v4j-2194200000-c5d437d80acf86fdf486 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00rf-9343000000-dfbe802e6925d43977e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-0009800000-e1fcc41ce51a5eff8deb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0005-0009000000-ccf153c7703c891eb1ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-0019000000-5bd9b8c892ce4a32df87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-920f9e6e9922687d5a0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0009300000-6d8ca177115c6a7900bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-1029000000-4e53cc76a5353b8f1205 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035174 |
|---|
| FooDB ID | FDB013814 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013864 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 59441520 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|