| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:55:46 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029098 |
|---|
| Identification |
|---|
| Common Name | Cinncassiol A |
|---|
| Class | Small Molecule |
|---|
| Description | Cinncassiol A is found in herbs and spices. Cinncassiol A is a constituent of Cinnamomum cassia (Chinese cinnamon) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
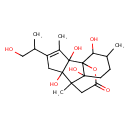
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sincassiol? | HMDB |
|
|---|
| Chemical Formula | C20H30O7 |
|---|
| Average Molecular Mass | 382.448 g/mol |
|---|
| Monoisotopic Mass | 382.199 g/mol |
|---|
| CAS Registry Number | 73599-11-8 |
|---|
| IUPAC Name | 2,6,8,12-tetrahydroxy-4-(1-hydroxypropan-2-yl)-3,7,11-trimethyl-13-oxatetracyclo[5.5.3.0¹,⁸.0²,⁶]pentadec-3-en-14-one |
|---|
| Traditional Name | 2,6,8,12-tetrahydroxy-4-(1-hydroxypropan-2-yl)-3,7,11-trimethyl-13-oxatetracyclo[5.5.3.0¹,⁸.0²,⁶]pentadec-3-en-14-one |
|---|
| SMILES | CC(CO)C1=C(C)C2(O)C(O)(C1)C1(C)CC(=O)OC22C(O)C(C)CCC12O |
|---|
| InChI Identifier | InChI=1S/C20H30O7/c1-10-5-6-17(24)16(4)8-14(22)27-20(17,15(10)23)19(26)12(3)13(11(2)9-21)7-18(16,19)25/h10-11,15,21,23-26H,5-9H2,1-4H3 |
|---|
| InChI Key | WIFHAKQJYHVTQK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as terpene lactones. These are prenol lipids containing a lactone ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Terpene lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Terpene lactone
- Sesquiterpenoid
- Caprolactone
- Delta valerolactone
- Delta_valerolactone
- Oxepane
- Oxane
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Oxacycle
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p9-1901000000-266ac3e3370a2a9182ef | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0bt9-4409035000-4064d21232ec9e245622 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0009000000-a4df3f1424c336bb8c19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-5019000000-3832c71e4410de0e6e66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pba-9002000000-512d4002a229120a5047 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-232437e781d49d3b0baf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gx0-1009000000-f8914a546bd2aa53ef34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9113000000-5846f6d99f672d0ac24c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-7f394c7458251fb3d2c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-3ae4a8058874c9cdad1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc1-0619000000-bfab011bc8a6ccfd57f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-33341a0b02e7805263c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0089-0069000000-ba4cfd18152cd0a8e40c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9225000000-722daa14f734e86d1f69 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035164 |
|---|
| FooDB ID | FDB013803 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013860 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12880072 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|