| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:55:22 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029090 |
|---|
| Identification |
|---|
| Common Name | Digeranyl |
|---|
| Class | Small Molecule |
|---|
| Description | Digeranyl is found in citrus. Digeranyl is isolated from bergamot oi |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
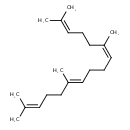
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,6E)-1,1'-Oxybis(3,7-dimethylocta-2,6-diene) | HMDB | | 1,1'-Oxybis(3,7-dimethyl-(2E,6E)-2,6-octadiene | HMDB | | Digeranyl ether | HMDB |
|
|---|
| Chemical Formula | C20H34 |
|---|
| Average Molecular Mass | 274.484 g/mol |
|---|
| Monoisotopic Mass | 274.266 g/mol |
|---|
| CAS Registry Number | 35162-77-7 |
|---|
| IUPAC Name | (6Z,10E)-2,6,11,15-tetramethylhexadeca-2,6,10,14-tetraene |
|---|
| Traditional Name | (6Z,10E)-2,6,11,15-tetramethylhexadeca-2,6,10,14-tetraene |
|---|
| SMILES | CC(C)=CCC\C(C)=C/CC\C=C(/C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C20H34/c1-17(2)11-9-15-19(5)13-7-8-14-20(6)16-10-12-18(3)4/h11-14H,7-10,15-16H2,1-6H3/b19-13-,20-14+ |
|---|
| InChI Key | UXUPDBJCOQWXPC-LRVMPXQBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic diterpenoids. These are diterpenoids (compounds made of four consecutive isoprene units) that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Acyclic diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic diterpenoid
- Alkatetraene
- Branched unsaturated hydrocarbon
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Acyclic olefin
- Hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ap0-5940000000-46cfffa76cbad5372149 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0290000000-6b173ad24068dd42fe64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar0-3970000000-62af8105a45338d7bb84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9620000000-00bea2672ae02befc532 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-d3ebc47987c37bd728b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-6d2f28a1082b15411613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-2980000000-f25c9e059decf66d3cf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-6d027560b1c478c2615d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-a8bc9d46ba4830f6ddd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-0890000000-e566b0f2d2fe32015a3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0059-4980000000-f7b6830d67419a5c26dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9720000000-a073ea20d2b74604b9eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o0-9500000000-c686768dcbee67cc84a5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035152 |
|---|
| FooDB ID | FDB013789 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00014485 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8554097 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10378654 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|