| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:55:16 UTC |
|---|
| Update Date | 2016-11-09 01:18:49 UTC |
|---|
| Accession Number | CHEM029087 |
|---|
| Identification |
|---|
| Common Name | Chrycorin |
|---|
| Class | Small Molecule |
|---|
| Description | Chrycorin is found in herbs and spices. Chrycorin is isolated from Chrysanthemum coronarium (chop-suey greens |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
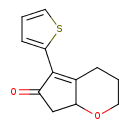
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4,7,7a-tetrahydro-5-(2-Thienyl)cyclopenta[b]pyran-6(2H)-one, 9ci | HMDB |
|
|---|
| Chemical Formula | C12H12O2S |
|---|
| Average Molecular Mass | 220.287 g/mol |
|---|
| Monoisotopic Mass | 220.056 g/mol |
|---|
| CAS Registry Number | 91362-90-2 |
|---|
| IUPAC Name | 5-(thiophen-2-yl)-2H,3H,4H,6H,7H,7aH-cyclopenta[b]pyran-6-one |
|---|
| Traditional Name | 5-(thiophen-2-yl)-2H,3H,4H,7H,7aH-cyclopenta[b]pyran-6-one |
|---|
| SMILES | O=C1CC2OCCCC2=C1C1=CC=CS1 |
|---|
| InChI Identifier | InChI=1S/C12H12O2S/c13-9-7-10-8(3-1-5-14-10)12(9)11-4-2-6-15-11/h2,4,6,10H,1,3,5,7H2 |
|---|
| InChI Key | FCVWHDAKNZMSON-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxanes. Oxanes are compounds containing an oxane (tetrahydropyran) ring, which is a six-member saturated aliphatic heterocycle with one oxygen atom and five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Oxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxane
- Heteroaromatic compound
- Thiophene
- Cyclic ketone
- Ketone
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-7910000000-c96ebff4cf053c60c575 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0190000000-d03ee57364d80a37cb56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9510000000-095a8e5f111213e9c738 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udu-9400000000-c6091ee79d4d42258926 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1190000000-0ad21a3888cfffb83f70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-8690000000-5c21a95435a6164aa937 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9400000000-eaeafdcd72c1aa5c23ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-ea659231e8550fd0dc62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0190000000-5fe4753e9a2369232060 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-0910000000-2106bcb39a94fabed43c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-8288cf3584dbbc915702 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2390000000-77799d201ee0a29735d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-4920000000-5b07cda6a4a9e32f437a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035149 |
|---|
| FooDB ID | FDB013786 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055327 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9610821 |
|---|
| ChEBI ID | 174112 |
|---|
| PubChem Compound ID | 11435957 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|