| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:53:17 UTC |
|---|
| Update Date | 2016-11-09 01:18:48 UTC |
|---|
| Accession Number | CHEM029052 |
|---|
| Identification |
|---|
| Common Name | (3beta,17alpha,23R)-17,23-Epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione |
|---|
| Class | Small Molecule |
|---|
| Description | (3beta,17alpha,23R)-17,23-Epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione is found in herbs and spices. (3beta,17alpha,23R)-17,23-Epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione is a constituent of Muscari comosum (tassel hyacinth) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
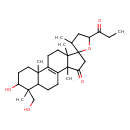
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3b,17a,23R)-17,23-Epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione | Generator | | (3Β,17α,23R)-17,23-epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione | Generator | | Madasiatate | HMDB |
|
|---|
| Chemical Formula | C29H44O5 |
|---|
| Average Molecular Mass | 472.657 g/mol |
|---|
| Monoisotopic Mass | 472.319 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5'-hydroxy-6'-(hydroxymethyl)-2',3,6',11',15'-pentamethyl-5-propanoylspiro[oxolane-2,14'-tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan]-1'(10')-en-12'-one |
|---|
| Traditional Name | 5'-hydroxy-6'-(hydroxymethyl)-2',3,6',11',15'-pentamethyl-5-propanoylspiro[oxolane-2,14'-tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan]-1'(10')-en-12'-one |
|---|
| SMILES | CCC(=O)C1CC(C)C2(CC(=O)C3(C)C4=C(CCC23C)C2(C)CCC(O)C(C)(CO)C2CC4)O1 |
|---|
| InChI Identifier | InChI=1S/C29H44O5/c1-7-20(31)21-14-17(2)29(34-21)15-24(33)28(6)19-8-9-22-25(3,18(19)10-13-27(28,29)5)12-11-23(32)26(22,4)16-30/h17,21-23,30,32H,7-16H2,1-6H3 |
|---|
| InChI Key | HJQFQTJAYYPCLS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- 3-hydroxysteroid
- Hydroxysteroid
- 15-oxosteroid
- Oxosteroid
- Steroid
- Cyclic alcohol
- Tetrahydrofuran
- Ketone
- Secondary alcohol
- Ether
- Dialkyl ether
- Organoheterocyclic compound
- Oxacycle
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-2113900000-5cb9b31088d4b7b546f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0zfr-5300395000-1265ed7fdfbc87d3ab17 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0001900000-fce48a6cb4168a60f131 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-1042900000-1cfda703b9138419e376 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0198000000-5d387d7400996e35534a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-4e55255dc5479f33a891 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fkc-1000900000-4fec62ab179e9e42f210 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2019300000-6b5956359a0ce54af336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-91947a5f3574d4c5169f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2003900000-7e7a79a3ebeeae74a068 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9001700000-b3af1745e7927cacabc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0002900000-d83e8d4e75459f98b76c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-0024900000-6c473e67e0921feb9a51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap3-9123800000-68622c7b7009df3b8779 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035112 |
|---|
| FooDB ID | FDB013743 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 16161196 |
|---|
| ChEBI ID | 168258 |
|---|
| PubChem Compound ID | 14634457 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|