| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:49:09 UTC |
|---|
| Update Date | 2016-11-09 01:18:47 UTC |
|---|
| Accession Number | CHEM028948 |
|---|
| Identification |
|---|
| Common Name | Xanthohumol D |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of chalcones that is trans-chalcone substituted by hydroxy groups at positions 4, 2' and 4', a methoxy group at position 6' and a 2-hydroxy-3-methylbut-3-en-1-yl group at position 3'. It has been isolated as a racemate from Humulus lupulus and has been shown to exhibit inhibitory activity against NO production. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
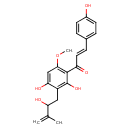
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-(2-Hydroxy-3-methylbutyl-3-enyl)-4,2',4'-trihydroxy-6'-methoxychalcone | ChEBI | | rac-(2E)-1-{2,4-dihydroxy-3-[2-hydroxy-3-methyl-3-but-3-enyl]-6-methoxyphenyl}-3-(4-hydroxyphenyl)-2-propen-1-one | ChEBI |
|
|---|
| Chemical Formula | C21H22O6 |
|---|
| Average Molecular Mass | 370.396 g/mol |
|---|
| Monoisotopic Mass | 370.142 g/mol |
|---|
| CAS Registry Number | 274675-25-1 |
|---|
| IUPAC Name | (2E)-1-[2,4-dihydroxy-3-(2-hydroxy-3-methylbut-3-en-1-yl)-6-methoxyphenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one |
|---|
| Traditional Name | xanthohumol D |
|---|
| SMILES | COC1=C(C(=O)\C=C\C2=CC=C(O)C=C2)C(O)=C(CC(O)C(C)=C)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H22O6/c1-12(2)17(24)10-15-18(25)11-19(27-3)20(21(15)26)16(23)9-6-13-4-7-14(22)8-5-13/h4-9,11,17,22,24-26H,1,10H2,2-3H3/b9-6+ |
|---|
| InChI Key | IIWLGOCXDBSFCM-RMKNXTFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-prenylated chalcones. These are chalcones featuring a C5-isoprenoid unit at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | 3-prenylated chalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-prenylated chalcone
- 2'-hydroxychalcone
- Cinnamylphenol
- Hydroxycinnamic acid or derivatives
- Methoxyphenol
- Phenoxy compound
- Benzoyl
- Phenol ether
- Resorcinol
- Styrene
- Aryl ketone
- Methoxybenzene
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Vinylogous acid
- Enone
- Secondary alcohol
- Ketone
- Ether
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fdp-9257000000-e93616bcdfc00d5f0eff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0006-4100159000-a73946662fee744891e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0129000000-f79c99b20767293c1063 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ds-9343000000-2a20e5aedac4bddb9324 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0l7i-6940000000-25b60ac9ed7eff1613a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0039000000-692ffe7dfab4150019f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0671-1493000000-424d244d7b00e13bc886 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-067i-9520000000-677eddaac31484f3246b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0019000000-11a1964c498d91ff1132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-1963000000-6e9da9f0627794e55667 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2910000000-a64df3dda5e77ae4f800 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-53e6b228c1fcbc5c164f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1749000000-2a4c83fe1b493b0ee475 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2933000000-c39e9b1fa1c7eef6c3d5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035003 |
|---|
| FooDB ID | FDB013607 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00014472 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7129 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8492534 |
|---|
| ChEBI ID | 66335 |
|---|
| PubChem Compound ID | 10317069 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|