| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:47:00 UTC |
|---|
| Update Date | 2016-11-09 01:18:47 UTC |
|---|
| Accession Number | CHEM028900 |
|---|
| Identification |
|---|
| Common Name | Geniposidic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Geniposidic acid is found in beverages. Geniposidic acid is a constituent of Genipa americana (genipap) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
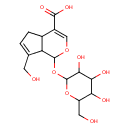
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Geniposidate | Generator | | 7-(Hydroxymethyl)-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4ah,5H,7ah-cyclopenta[c]pyran-4-carboxylate | HMDB |
|
|---|
| Chemical Formula | C16H22O10 |
|---|
| Average Molecular Mass | 374.340 g/mol |
|---|
| Monoisotopic Mass | 374.121 g/mol |
|---|
| CAS Registry Number | 27741-01-1 |
|---|
| IUPAC Name | 7-(hydroxymethyl)-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,7aH-cyclopenta[c]pyran-4-carboxylic acid |
|---|
| Traditional Name | 7-(hydroxymethyl)-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,7aH-cyclopenta[c]pyran-4-carboxylic acid |
|---|
| SMILES | OCC1OC(OC2OC=C(C3CC=C(CO)C23)C(O)=O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C16H22O10/c17-3-6-1-2-7-8(14(22)23)5-24-15(10(6)7)26-16-13(21)12(20)11(19)9(4-18)25-16/h1,5,7,9-13,15-21H,2-4H2,(H,22,23) |
|---|
| InChI Key | ZJDOESGVOWAULF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as iridoid o-glycosides. These are iridoid monoterpenes containing a glycosyl (usually a pyranosyl) moiety linked to the iridoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Iridoid O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Iridoid o-glycoside
- Hexose monosaccharide
- Glycosyl compound
- Iridoid-skeleton
- O-glycosyl compound
- Bicyclic monoterpenoid
- Monoterpenoid
- Monosaccharide
- Oxane
- Vinylogous ester
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Polyol
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052e-9425000000-fca03c21c13c3909d3cf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0002-2723049000-90b44f7f5259a1d0772d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fs-0926000000-45cf92e48bd8c9f6527b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0902000000-0b93ac9eafd9092b1e6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-4900000000-b722af45f3ca08684d75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03kc-1549000000-b1fa7aaadb1b0df619b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-1923000000-eafd7897025d63b2f572 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-5920000000-476c6ea1f06811b2b456 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0903000000-89357f5dfb54770b5f52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0900000000-7475fe3ceb9d0d08f6d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0avi-5900000000-6d5c216d7f3eb973350e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0209000000-19bcaa18e2fa7411858b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0629-5945000000-586c9397440e29ba2331 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5920000000-2ff91c396d00168d9f62 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034942 |
|---|
| FooDB ID | FDB013535 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010564 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Geniposidic acid |
|---|
| Chemspider ID | 286285 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 323273 |
|---|
| Kegg Compound ID | C11673 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|