| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:46:57 UTC |
|---|
| Update Date | 2016-11-09 01:18:47 UTC |
|---|
| Accession Number | CHEM028899 |
|---|
| Identification |
|---|
| Common Name | Artabsinolide D |
|---|
| Class | Small Molecule |
|---|
| Description | Artabsinolide C is found in alcoholic beverages. Artabsinolide C is a constituent of Artemisia absinthium (wormwood) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
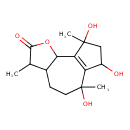
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H22O5 |
|---|
| Average Molecular Mass | 282.332 g/mol |
|---|
| Monoisotopic Mass | 282.147 g/mol |
|---|
| CAS Registry Number | 81907-04-2 |
|---|
| IUPAC Name | 6,7,9-trihydroxy-3,6,9-trimethyl-2H,3H,3aH,4H,5H,6H,7H,8H,9H,9bH-azuleno[4,5-b]furan-2-one |
|---|
| Traditional Name | 6,7,9-trihydroxy-3,6,9-trimethyl-3H,3aH,4H,5H,7H,8H,9bH-azuleno[4,5-b]furan-2-one |
|---|
| SMILES | CC1C2CCC(C)(O)C3=C(C2OC1=O)C(C)(O)CC3O |
|---|
| InChI Identifier | InChI=1S/C15H22O5/c1-7-8-4-5-14(2,18)10-9(16)6-15(3,19)11(10)12(8)20-13(7)17/h7-9,12,16,18-19H,4-6H2,1-3H3 |
|---|
| InChI Key | YZKOCDPFUHYTHX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma butyrolactone
- Tertiary alcohol
- Tetrahydrofuran
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Polyol
- Oxacycle
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0300-2390000000-513c4050baec4130c054 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-06ur-2001900000-3b8c2d72d4c73aa324fb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0090000000-5341ada221d2bcd680bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0490000000-dd52683136ad4324f3b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-1690000000-00998b38749377e26102 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-59695e8e7944ac744b01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0090000000-94c8a9a0a573a381dae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-9520000000-a6e03a6dc019688bbdc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-4206ab54dd729eeefe27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-ae18ad965d4fbc7a2fc2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2890000000-67a599088e989225b01d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-1c245b7d9de3be531223 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00li-1290000000-6e4c59e5f3e9a3b8f4d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g0-1920000000-973cacbb39b81e86edca | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034941 |
|---|
| FooDB ID | FDB013534 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020869 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013799 |
|---|
| ChEBI ID | 174699 |
|---|
| PubChem Compound ID | 131751640 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|