| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:44:57 UTC |
|---|
| Update Date | 2016-11-09 01:18:46 UTC |
|---|
| Accession Number | CHEM028849 |
|---|
| Identification |
|---|
| Common Name | 3alpha,4,7,7alpha-Tetrahydro-4-hydroxy-1H-isoindole-1,3(2H)-dione |
|---|
| Class | Small Molecule |
|---|
| Description | 3alpha,4,7,7alpha-Tetrahydro-4-hydroxy-1H-isoindole-1,3(2H)-dione is found in milk and milk products. Potential food contaminant arising from the metabolism of Captan CXH76-I. 3alpha,4,7,7alpha-Tetrahydro-4-hydroxy-1H-isoindole-1,3(2H)-dione is detected in mil |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
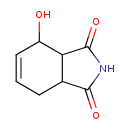
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3a,4,7,7a-Tetrahydro-4-hydroxy-1H-isoindole-1,3(2H)-dione | Generator | | 3Α,4,7,7α-tetrahydro-4-hydroxy-1H-isoindole-1,3(2H)-dione | Generator | | 1,2,3,6-tetrahydro-3-Hydroxyphthalimide | HMDB |
|
|---|
| Chemical Formula | C8H9NO3 |
|---|
| Average Molecular Mass | 167.162 g/mol |
|---|
| Monoisotopic Mass | 167.058 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4-hydroxy-2,3,3a,4,7,7a-hexahydro-1H-isoindole-1,3-dione |
|---|
| Traditional Name | 4-hydroxy-3a,4,7,7a-tetrahydro-2H-isoindole-1,3-dione |
|---|
| SMILES | OC1C=CCC2C1C(=O)NC2=O |
|---|
| InChI Identifier | InChI=1S/C8H9NO3/c10-5-3-1-2-4-6(5)8(12)9-7(4)11/h1,3-6,10H,2H2,(H,9,11,12) |
|---|
| InChI Key | MLJWDNXRMUBJJU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoindolones. These are aromatic polycyclic compounds that an isoindole bearing a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoindoles and derivatives |
|---|
| Sub Class | Isoindolines |
|---|
| Direct Parent | Isoindolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoindolone
- Isoindole
- Pyrroline
- Secondary alcohol
- N-acylimine
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Alcohol
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-8900000000-22dfe71e7ae98a016058 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fk9-9630000000-0a8a836a2c1abd182eba | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0900000000-2d45e08f7d6290954308 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-3900000000-5f05e4e9532c2c88f68a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9400000000-6014e90e816e2054ed70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-f165e280feaed788cf4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-7900000000-b8013b95abf5a3e1be32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-346a8e648325dd7abfc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-54ab2df4743f6d0ea000 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014m-5900000000-f3dae057be31e08cdd14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-8c7f30426b820fbc4f33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-02f8857feda43cbfeef2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1900000000-6961f31dae12cc01783f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f97-9300000000-9541c027eb5ade49cd69 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034889 |
|---|
| FooDB ID | FDB013465 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013793 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 86242667 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|