| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:44:45 UTC |
|---|
| Update Date | 2016-11-09 01:18:46 UTC |
|---|
| Accession Number | CHEM028843 |
|---|
| Identification |
|---|
| Common Name | 3-Carboxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-propanoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 3-Carboxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-propanoic acid is found in alcoholic beverages. 3-Carboxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-propanoic acid is present in soy and worcester sauces, yeast extract and wine as the (3S)-diastereoisomers. 3-Carboxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-propanoic acid is formed by Pictet-Spengler condensation of tryptophan with 4-oxobutanoic acid to give predominantly the cis-isomer |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
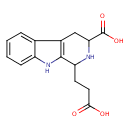
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Carboxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-propanoate | Generator | | 1-(2-Carboxyethyl)-1,2,3,4-tetrahydro-b-carboline-3-carboxylic acid | HMDB | | 1-(2-Carboxyethyl)-1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylate | Generator |
|
|---|
| Chemical Formula | C15H16N2O4 |
|---|
| Average Molecular Mass | 288.299 g/mol |
|---|
| Monoisotopic Mass | 288.111 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1-(2-carboxyethyl)-1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylic acid |
|---|
| Traditional Name | 1-(2-carboxyethyl)-1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylic acid |
|---|
| SMILES | OC(=O)CCC1NC(CC2=C1NC1=CC=CC=C21)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H16N2O4/c18-13(19)6-5-11-14-9(7-12(16-11)15(20)21)8-3-1-2-4-10(8)17-14/h1-4,11-12,16-17H,5-7H2,(H,18,19)(H,20,21) |
|---|
| InChI Key | PPKGNUKJFFAWHY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harman
- Beta-carboline
- Pyridoindole
- Alpha-amino acid
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- Aralkylamine
- Dicarboxylic acid or derivatives
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kf-3690000000-8a203209ee26cc9d81b2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01bi-9427100000-83bbdc724a92bedb9dd9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-007c-0090000000-c072e5e5207b8c910fb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0036-0590000000-b615c774781c63631475 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001j-1900000000-2b7cf789bff4868649b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-016a1ced5998391f910d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000f-0290000000-f825e5f837e939c27da6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-8980000000-5333ae5b5ce67dd5e54d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0290000000-993c9e1d7e7cf2f7fc90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mn-0790000000-ad5581853fcf6bba5c6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00mn-2920000000-a796fcd75cb858a9a779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-19de8b5ab0e62272a9e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-007c-0190000000-eab8f030770e8aaaba37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001m-1930000000-765209de6319e55c489e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034881 |
|---|
| FooDB ID | FDB013456 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013791 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751635 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01492 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|