| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:44:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:46 UTC |
|---|
| Accession Number | CHEM028842 |
|---|
| Identification |
|---|
| Common Name | 1-[(5-Amino-5-carboxypentyl)amino]-1-deoxyfructose |
|---|
| Class | Small Molecule |
|---|
| Description | 1-[(5-Amino-5-carboxypentyl)amino]-1-deoxyfructose is found in milk and milk products. Amadori rearrangement produced found in heated milk and other foodstuff |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
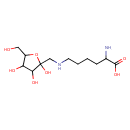
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| e-Deoxyfructosyllysine | HMDB | | e-Fructoselysine | HMDB | | epsilon-Fructoselysine | HMDB | | Fructose lysine | HMDB | | Fructosyl-lysine | HMDB | | Fructosyllysine | HMDB | | N6-(1-Deoxy-D-fructos-1-yl)-L-lysine | HMDB | | N6-(1-Deoxyfructos-1-yl)lysine | HMDB |
|
|---|
| Chemical Formula | C12H24N2O7 |
|---|
| Average Molecular Mass | 308.328 g/mol |
|---|
| Monoisotopic Mass | 308.158 g/mol |
|---|
| CAS Registry Number | 21291-40-7 |
|---|
| IUPAC Name | 2-amino-6-({[2,3,4-trihydroxy-5-(hydroxymethyl)oxolan-2-yl]methyl}amino)hexanoic acid |
|---|
| Traditional Name | 2-amino-6-({[2,3,4-trihydroxy-5-(hydroxymethyl)oxolan-2-yl]methyl}amino)hexanoic acid |
|---|
| SMILES | NC(CCCCNCC1(O)OC(CO)C(O)C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C12H24N2O7/c13-7(11(18)19)3-1-2-4-14-6-12(20)10(17)9(16)8(5-15)21-12/h7-10,14-17,20H,1-6,13H2,(H,18,19) |
|---|
| InChI Key | ZAWLGBRDVQURAK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Pentose monosaccharide
- Medium-chain fatty acid
- Amino fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Monosaccharide
- Tetrahydrofuran
- Amino acid or derivatives
- Amino acid
- Hemiacetal
- Secondary alcohol
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Organoheterocyclic compound
- Secondary amine
- Monocarboxylic acid or derivatives
- Polyol
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organic oxide
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052o-9330000000-0a3b774a2099aa1841b2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-0udi-3921357000-15c6c7c5a1db6a51e48e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0btc-1793000000-7d5e15faa2663e22b446 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ea-4590000000-b16d04d9d276a05e3ff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-9700000000-7c1717d0cdc5f3d13746 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-5449000000-5d96bc69a2ef7283d4e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-4973000000-7fa6ef2bd5ebb8d2556f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-c189fd0a336d3d4ad871 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0abc-0094000000-d77f3d23caad13a312c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-2982000000-9c19ee079047920ca852 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003r-5900000000-01fbe3f994d9a06dd7dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0039000000-45b8040e84722234d782 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-1494000000-28cab6b1988ba64e481a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-9410000000-ecdb35da557e9f2d2748 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034879 |
|---|
| FooDB ID | FDB013454 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013790 |
|---|
| ChEBI ID | 24109 |
|---|
| PubChem Compound ID | 131751634 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|