| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:44:14 UTC |
|---|
| Update Date | 2016-11-09 01:18:46 UTC |
|---|
| Accession Number | CHEM028832 |
|---|
| Identification |
|---|
| Common Name | Xanthohumol B |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of chalcones that is (2E)-1-(3,4-dihydro-2H-chromen-6-yl)-3-(4-hydroxyphenyl)prop-2-en-1-one substituted by hydroxy groups at position 3 and 5, a methoxy group at position 7 and geminal methyl groups at position 2. It has been isolated as a racemate from Humulus lupulus and has been shown to exhibit inhibitory activity against nitric oxide production. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
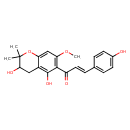
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dehydrocycloxanthohumol hydrate | ChEBI | | rac-(e)-1-(3,5-Dihydroxy-7-methoxy-2,2-dimethyl-3,4-dihydrochromen-6-yl)-3-(4-hydroxyphenyl)prop-2-en-1-one | ChEBI | | rac-6'',6''-Dimethyl-5''-hydroxy-4'',5''-dihydropyrano[2'',3'':4',3']-4,2'-dihydroxy-6'-methoxychalcone | ChEBI | | rac-6-[3,4-Dihydro-3,5-dihydroxy-7-methoxy-2,2-dimethyl-2H-benzo[b]pyrano]-3-(4-hydroxyphenyl)-2-propen-1-one | ChEBI | | Dehydrocycloxanthohumol hydric acid | Generator | | 6'',6''-Dimethyl-5''-hydroxy-4'',5''-dihydropyrano[2'',3'':4',3']-4,2'-dihydroxy-6'-methoxychalcone | HMDB |
|
|---|
| Chemical Formula | C21H22O6 |
|---|
| Average Molecular Mass | 370.396 g/mol |
|---|
| Monoisotopic Mass | 370.142 g/mol |
|---|
| CAS Registry Number | 189308-10-9 |
|---|
| IUPAC Name | (2E)-1-(3,5-dihydroxy-7-methoxy-2,2-dimethyl-3,4-dihydro-2H-1-benzopyran-6-yl)-3-(4-hydroxyphenyl)prop-2-en-1-one |
|---|
| Traditional Name | xanthohumol B |
|---|
| SMILES | COC1=C(C(=O)\C=C\C2=CC=C(O)C=C2)C(O)=C2CC(O)C(C)(C)OC2=C1 |
|---|
| InChI Identifier | InChI=1S/C21H22O6/c1-21(2)18(24)10-14-16(27-21)11-17(26-3)19(20(14)25)15(23)9-6-12-4-7-13(22)8-5-12/h4-9,11,18,22,24-25H,10H2,1-3H3/b9-6+ |
|---|
| InChI Key | GUQGMEWOCKDLDE-RMKNXTFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2'-hydroxychalcones. These are organic compounds containing chalcone skeleton that carries a hydroxyl group at the 2'-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | 2'-Hydroxychalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2'-hydroxychalcone
- Hydroxycinnamic acid or derivatives
- 2,2-dimethyl-1-benzopyran
- Chromane
- Benzopyran
- 1-benzopyran
- Anisole
- Styrene
- Aryl ketone
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous acid
- Alpha,beta-unsaturated ketone
- Acryloyl-group
- Enone
- Secondary alcohol
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Ether
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udl-0229000000-b811f90951d1a0a835d1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-6040190000-bdc2a7531e971d5f1a9d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0179000000-e2301b1431f4b57e5b7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0493000000-6468906cb55db17ec732 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2940000000-8c2ed436d015fefe3a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0149000000-423b4abd708a051724ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xs-6594000000-22d370d3f79419763d48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3590000000-430efc544d480953d59b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-0be4f2f0792fa82b11b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0898000000-a197e8c9c81ccea5ceca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1930000000-bf324f554727d9051fa0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0019000000-7b119b59c36247b7ed5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-1391000000-92e5e602575e67980af7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00su-4962000000-6aa1110047dcb2f70570 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034867 |
|---|
| FooDB ID | FDB013439 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00014478 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7127 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7975271 |
|---|
| ChEBI ID | 66334 |
|---|
| PubChem Compound ID | 9799506 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|