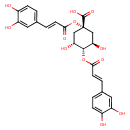| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:41:31 UTC |
|---|
| Update Date | 2016-11-09 01:18:45 UTC |
|---|
| Accession Number | CHEM028777 |
|---|
| Identification |
|---|
| Common Name | 1,4-Di-O-caffeoylquinic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 1,4-Di-O-caffeoylquinic acid is found in herbs and spices. 1,4-Di-O-caffeoylquinic acid is isolated from flowers and leaves of Helichrysum italicum (curry plant |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Di-O-caffeoylquinate | Generator | | 1,4-Bis-(3,4-dihydroxycinnamoyl)quinic acid | HMDB | | 1,4-Dicaffeoylquinic acid | HMDB | | 1,4-Dicaffeylquinic acid | HMDB | | NSC 91529 | HMDB | | (1S,3R,4S,5R)-1,4-Bis({[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy})-3,5-dihydroxycyclohexane-1-carboxylate | Generator |
|
|---|
| Chemical Formula | C25H24O12 |
|---|
| Average Molecular Mass | 516.451 g/mol |
|---|
| Monoisotopic Mass | 516.127 g/mol |
|---|
| CAS Registry Number | 1182-34-9 |
|---|
| IUPAC Name | (1S,3R,4S,5R)-1,4-bis({[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy})-3,5-dihydroxycyclohexane-1-carboxylic acid |
|---|
| Traditional Name | (1S,3R,4S,5R)-1,4-bis({[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy})-3,5-dihydroxycyclohexane-1-carboxylic acid |
|---|
| SMILES | O[C@@H]1C[C@](C(=O)O)(C[C@@H](O)[C@H]1OC(=O)\C=C\C1=CC=C(O)C(O)=C1)OC(=O)\C=C\C1=CC=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(32)36-23-19(30)11-25(24(34)35,12-20(23)31)37-22(33)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-31H,11-12H2,(H,34,35)/b7-3+,8-4+/t19-,20-,23-,25+/m1/s1 |
|---|
| InChI Key | IYXQRCXQQWUFQV-PSEXTPKNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinic acids and derivatives. Quinic acids and derivatives are compounds containing a quinic acid moiety (or a derivative thereof), which is a cyclitol made up of a cyclohexane ring that bears four hydroxyl groups at positions 1,3.4, and 5, as well as a carboxylic acid at position 1. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Quinic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinic acid
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Hydroxycinnamic acid or derivatives
- Cinnamic acid ester
- Tricarboxylic acid or derivatives
- Catechol
- Styrene
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Fatty acid ester
- Cyclohexanol
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Fatty acyl
- Monocyclic benzene moiety
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03du-6894100000-3cc776275036e1452e7a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01pa-5947105000-48f4ae26dfb0f19719bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02tj-0609440000-218969ac8eca3c447140 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ri-0908300000-56da112fdf3b7309f1d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-070f-0912100000-086a2d351f0d8ecd09c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gb9-0319470000-990109b660542b831997 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kk9-0938200000-bb497a7bc6d677084f2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-0902000000-5be08bec609d75adc06f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-b378e9bbf66017fd276b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0673-0529440000-2003b2a4a48754b76d17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0901100000-b35e0e1b7e8dada58276 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-044j-0914840000-577fddcfd51d555648b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-0930200000-01988e78a39b9187e866 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01rg-3921600000-38b4c7191e0ee9562793 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034766 |
|---|
| FooDB ID | FDB013317 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055091 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 22912997 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|