| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 00:40:44 UTC |
|---|
| Update Date | 2016-11-09 01:18:45 UTC |
|---|
| Accession Number | CHEM028760 |
|---|
| Identification |
|---|
| Common Name | Icariside E5 |
|---|
| Class | Small Molecule |
|---|
| Description | Icariside E5 is found in fruits. Icariside E5 is a constituent of ripe fruits of Capsicum annuum var. acuminatum |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H34O11 |
|---|
| Average Molecular Mass | 522.542 g/mol |
|---|
| Monoisotopic Mass | 522.210 g/mol |
|---|
| CAS Registry Number | 126176-79-2 |
|---|
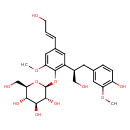
| IUPAC Name | (2S,3R,4S,5S,6R)-2-{2-[(2R)-1-hydroxy-3-(4-hydroxy-3-methoxyphenyl)propan-2-yl]-4-[(1E)-3-hydroxyprop-1-en-1-yl]-6-methoxyphenoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | (2S,3R,4S,5S,6R)-2-{2-[(2R)-1-hydroxy-3-(4-hydroxy-3-methoxyphenyl)propan-2-yl]-4-[(1E)-3-hydroxyprop-1-en-1-yl]-6-methoxyphenoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | COC1=CC(\C=C/CO)=CC(C(CO)CC2=CC(OC)=C(O)C=C2)=C1OC1OC(CO)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C26H34O11/c1-34-19-10-15(5-6-18(19)30)8-16(12-28)17-9-14(4-3-7-27)11-20(35-2)25(17)37-26-24(33)23(32)22(31)21(13-29)36-26/h3-6,9-11,16,21-24,26-33H,7-8,12-13H2,1-2H3/b4-3- |
|---|
| InChI Key | UFFRBCKYXMEITK-ARJAWSKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbene glycosides. Stilbene glycosides are compounds structurally characterized by the presence of a carbohydrate moiety glycosidically linked to the stilbene skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Stilbene glycosides |
|---|
| Direct Parent | Stilbene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene glycoside
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Cinnamyl alcohol
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Styrene
- Phenol ether
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Monosaccharide
- Oxane
- Secondary alcohol
- Organoheterocyclic compound
- Ether
- Oxacycle
- Acetal
- Polyol
- Organic oxygen compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06rl-6702940000-84cd5792deec24c86261 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0btc-0109360000-4cd1c0a5ef55d8978a9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0209100000-ff154f1535e6044b2be9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0219000000-8224fcb99f1dadd53a1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-0307390000-b398abea3a57daa03ddc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-0209310000-a53d20844c65a906669b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054o-1119000000-3983e154af12e734ed78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000190000-f70201d8ecb357cb3cbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0200-0000930000-9b20e2f86465c723bc07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-1029220000-aaff05af103358d4d97f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-0219120000-246b51d5df634b36c1db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0905430000-07129bf726d0a28a2ad9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-2906210000-b0f5c23f7b451c350f90 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034749 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056763 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 28424399 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91884923 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|